+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5y3s | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human NLRP1 leucine rich repeat domain | ||||||
 Components Components | NACHT, LRR and PYD domains-containing protein 1 | ||||||
 Keywords Keywords | IMMUNE SYSTEM / inflammasome | ||||||
| Function / homology |  Function and homology information Function and homology informationNLRP1 inflammasome complex / NLRP1 inflammasome complex assembly / The NLRP1 inflammasome / self proteolysis / NLRP3 inflammasome complex / cysteine-type endopeptidase activator activity / Hydrolases; Acting on peptide bonds (peptidases) / pattern recognition receptor signaling pathway / cellular response to UV-B / cysteine-type endopeptidase activator activity involved in apoptotic process ...NLRP1 inflammasome complex / NLRP1 inflammasome complex assembly / The NLRP1 inflammasome / self proteolysis / NLRP3 inflammasome complex / cysteine-type endopeptidase activator activity / Hydrolases; Acting on peptide bonds (peptidases) / pattern recognition receptor signaling pathway / cellular response to UV-B / cysteine-type endopeptidase activator activity involved in apoptotic process / pattern recognition receptor activity / response to muramyl dipeptide / pyroptotic inflammatory response / signaling adaptor activity / antiviral innate immune response / activation of innate immune response / intrinsic apoptotic signaling pathway / positive regulation of interleukin-1 beta production / molecular condensate scaffold activity / protein homooligomerization / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / positive regulation of inflammatory response / peptidase activity / double-stranded RNA binding / regulation of inflammatory response / neuron apoptotic process / double-stranded DNA binding / regulation of apoptotic process / defense response to virus / defense response to bacterium / inflammatory response / protein domain specific binding / apoptotic process / nucleolus / enzyme binding / signal transduction / ATP hydrolysis activity / nucleoplasm / ATP binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.451 Å MOLECULAR REPLACEMENT / Resolution: 2.451 Å | ||||||
 Authors Authors | Jin, T. / Xiao, T.S. | ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Crystal structure of human NLRP1 LRR domain Authors: Jin, T. / Xiao, T.S. | ||||||
| History |
|
- Structure visualization
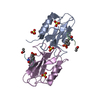
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5y3s.cif.gz 5y3s.cif.gz | 163.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5y3s.ent.gz pdb5y3s.ent.gz | 128.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5y3s.json.gz 5y3s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/y3/5y3s https://data.pdbj.org/pub/pdb/validation_reports/y3/5y3s ftp://data.pdbj.org/pub/pdb/validation_reports/y3/5y3s ftp://data.pdbj.org/pub/pdb/validation_reports/y3/5y3s | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1dfjS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 22790.402 Da / Num. of mol.: 4 / Fragment: UNP residues 790-990 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: NLRP1, CARD7, DEFCAP, KIAA0926, NAC, NALP1 / Production host: Homo sapiens (human) / Gene: NLRP1, CARD7, DEFCAP, KIAA0926, NAC, NALP1 / Production host:  #2: Chemical | #3: Chemical | ChemComp-NA / | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.09 Å3/Da / Density % sol: 60.25 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 6.4 Details: 1.6M Sodium Potassium Phosphate, 100mM HEPES, pH 6.4 |
-Data collection
| Diffraction | Mean temperature: 190 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 0.9 Å / Beamline: 22-ID / Wavelength: 0.9 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Nov 20, 2008 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 2.451→33.76 Å / Num. obs: 41422 / % possible obs: 98.9 % / Redundancy: 5.4 % / CC1/2: 0.999 / Rmerge(I) obs: 0.0572 / Rpim(I) all: 0.0268 / Net I/σ(I): 18.99 |
| Reflection shell | Resolution: 2.451→2.539 Å / Rmerge(I) obs: 0.9533 / CC1/2: 0.678 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1DFJ Resolution: 2.451→33.76 Å / SU ML: 0.33 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 28.82
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.451→33.76 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj













