[English] 日本語
 Yorodumi
Yorodumi- PDB-5xnc: Crystal structure of the branched-chain polyamine synthase (BpsA)... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5xnc | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the branched-chain polyamine synthase (BpsA) in complex with N4-aminopropylspermidine and 5-methylthioadenosine | ||||||
 Components Components | N(4)-bis(aminopropyl)spermidine synthase | ||||||
 Keywords Keywords | TRANSFERASE / Polyamine biosynthesis / spermidine / N4-aminopropylspermidine / Branched polyamines | ||||||
| Function / homology |  Function and homology information Function and homology informationN4-bis(aminopropyl)spermidine synthase / polyamine biosynthetic process / transferase activity, transferring alkyl or aryl (other than methyl) groups / transferase activity / cytoplasm Similarity search - Function | ||||||
| Biological species |   Thermococcus kodakarensis (archaea) Thermococcus kodakarensis (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.84 Å MOLECULAR REPLACEMENT / Resolution: 1.84 Å | ||||||
 Authors Authors | Mizohata, E. / Tse, K.M. / Fujita, J. / Inoue, T. | ||||||
 Citation Citation |  Journal: FEBS J. / Year: 2017 Journal: FEBS J. / Year: 2017Title: Active site geometry of a novel aminopropyltransferase for biosynthesis of hyperthermophile-specific branched-chain polyamine. Authors: Hidese, R. / Tse, K.M. / Kimura, S. / Mizohata, E. / Fujita, J. / Horai, Y. / Umezawa, N. / Higuchi, T. / Niitsu, M. / Oshima, T. / Imanaka, T. / Inoue, T. / Fujiwara, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5xnc.cif.gz 5xnc.cif.gz | 1014.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5xnc.ent.gz pdb5xnc.ent.gz | 847 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5xnc.json.gz 5xnc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5xnc_validation.pdf.gz 5xnc_validation.pdf.gz | 3.4 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5xnc_full_validation.pdf.gz 5xnc_full_validation.pdf.gz | 3.4 MB | Display | |
| Data in XML |  5xnc_validation.xml.gz 5xnc_validation.xml.gz | 103.6 KB | Display | |
| Data in CIF |  5xnc_validation.cif.gz 5xnc_validation.cif.gz | 142.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xn/5xnc https://data.pdbj.org/pub/pdb/validation_reports/xn/5xnc ftp://data.pdbj.org/pub/pdb/validation_reports/xn/5xnc ftp://data.pdbj.org/pub/pdb/validation_reports/xn/5xnc | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components on special symmetry positions |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Ens-ID: 1 / Beg auth comp-ID: GLU / Beg label comp-ID: GLU / End auth comp-ID: GLN / End label comp-ID: GLN / Refine code: 5 / Auth seq-ID: 10 - 340 / Label seq-ID: 30 - 360
NCS oper:
|
- Components
Components
-Protein , 1 types, 7 molecules ABCDEFG
| #1: Protein | Mass: 42445.941 Da / Num. of mol.: 7 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Thermococcus kodakarensis (strain ATCC BAA-918 / JCM 12380 / KOD1) (archaea) Thermococcus kodakarensis (strain ATCC BAA-918 / JCM 12380 / KOD1) (archaea)Strain: ATCC BAA-918 / JCM 12380 / KOD1 / Gene: bpsA, TK1691 / Plasmid: pET28a Details (production host): N-terminal HisTag/thrombin digestion site Production host:  References: UniProt: Q5JIZ3, N4-bis(aminopropyl)spermidine synthase |
|---|
-Non-polymers , 7 types, 1089 molecules 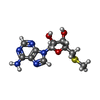
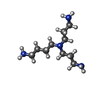











| #2: Chemical | ChemComp-MTA / #3: Chemical | ChemComp-N4P / #4: Chemical | ChemComp-GOL / #5: Chemical | ChemComp-FE / #6: Chemical | #7: Chemical | #8: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.37 Å3/Da / Density % sol: 48.08 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 5.6 Details: 100 mM sodium citrate tribasic dihydrate (pH 5.6), 2.5 M (w/v) 1,6-hexanediol Temp details: 4 degree C |
-Data collection
| Diffraction | Mean temperature: 100 K / Ambient temp details: cryogenic temperature |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL44XU / Wavelength: 0.9 Å / Beamline: BL44XU / Wavelength: 0.9 Å |
| Detector | Type: RAYONIX MX300HE / Detector: CCD / Date: Jun 30, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 1.84→50 Å / Num. obs: 241983 / % possible obs: 99.8 % / Redundancy: 5.2 % / Rsym value: 0.061 / Net I/σ(I): 27.7 |
| Reflection shell | Resolution: 1.84→1.91 Å / Redundancy: 5.2 % / Mean I/σ(I) obs: 2.8 / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.84→43.53 Å / Cor.coef. Fo:Fc: 0.975 / Cor.coef. Fo:Fc free: 0.959 / SU B: 7.167 / SU ML: 0.102 / Cross valid method: THROUGHOUT / ESU R: 0.117 / ESU R Free: 0.118 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS MOLECULAR REPLACEMENT / Resolution: 1.84→43.53 Å / Cor.coef. Fo:Fc: 0.975 / Cor.coef. Fo:Fc free: 0.959 / SU B: 7.167 / SU ML: 0.102 / Cross valid method: THROUGHOUT / ESU R: 0.117 / ESU R Free: 0.118 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45.03 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.84→43.53 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj




