+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5nc5 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of AcrBZ in complex with antibiotic puromycin | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSPORT PROTEIN / Membrane transporter / Multidrug efflux pump | ||||||
| Function / homology |  Function and homology information Function and homology informationalkane transmembrane transporter activity / alkane transport / Antimicrobial resistance / Iron assimilation using enterobactin / enterobactin transport / enterobactin transmembrane transporter activity / xenobiotic detoxification by transmembrane export across the cell outer membrane / periplasmic side of plasma membrane / efflux pump complex / xenobiotic detoxification by transmembrane export across the plasma membrane ...alkane transmembrane transporter activity / alkane transport / Antimicrobial resistance / Iron assimilation using enterobactin / enterobactin transport / enterobactin transmembrane transporter activity / xenobiotic detoxification by transmembrane export across the cell outer membrane / periplasmic side of plasma membrane / efflux pump complex / xenobiotic detoxification by transmembrane export across the plasma membrane / bile acid transmembrane transporter activity / Secretion of toxins / bile acid and bile salt transport / efflux transmembrane transporter activity / xenobiotic transmembrane transporter activity / xenobiotic transport / fatty acid transport / response to toxic substance / response to xenobiotic stimulus / response to antibiotic / identical protein binding / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |    | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.2 Å MOLECULAR REPLACEMENT / Resolution: 3.2 Å | ||||||
 Authors Authors | Du, D. / Luisi, B. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: Elife / Year: 2017 Journal: Elife / Year: 2017Title: An allosteric transport mechanism for the AcrAB-TolC multidrug efflux pump. Authors: Zhao Wang / Guizhen Fan / Corey F Hryc / James N Blaza / Irina I Serysheva / Michael F Schmid / Wah Chiu / Ben F Luisi / Dijun Du /   Abstract: Bacterial efflux pumps confer multidrug resistance by transporting diverse antibiotics from the cell. In Gram-negative bacteria, some of these pumps form multi-protein assemblies that span the cell ...Bacterial efflux pumps confer multidrug resistance by transporting diverse antibiotics from the cell. In Gram-negative bacteria, some of these pumps form multi-protein assemblies that span the cell envelope. Here, we report the near-atomic resolution cryoEM structures of the AcrAB-TolC multidrug efflux pump in resting and drug transport states, revealing a quaternary structural switch that allosterically couples and synchronizes initial ligand binding with channel opening. Within the transport-activated state, the channel remains open even though the pump cycles through three distinct conformations. Collectively, our data provide a dynamic mechanism for the assembly and operation of the AcrAB-TolC pump. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5nc5.cif.gz 5nc5.cif.gz | 1.3 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5nc5.ent.gz pdb5nc5.ent.gz | 1.1 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5nc5.json.gz 5nc5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nc/5nc5 https://data.pdbj.org/pub/pdb/validation_reports/nc/5nc5 ftp://data.pdbj.org/pub/pdb/validation_reports/nc/5nc5 ftp://data.pdbj.org/pub/pdb/validation_reports/nc/5nc5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3636C  8636C  8640C  5ng5C  5o66C  5v5sC  4dx5S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Multidrug efflux pump ... , 2 types, 6 molecules ABCFGH
| #1: Protein | Mass: 113665.180 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #3: Protein/peptide | Mass: 5304.423 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Protein / Sugars , 2 types, 5 molecules DE

| #2: Protein | Mass: 18317.566 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #4: Sugar | |
|---|
-Non-polymers , 6 types, 148 molecules 

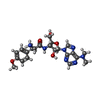






| #5: Chemical | ChemComp-D12 / #6: Chemical | ChemComp-D10 / #7: Chemical | ChemComp-DD9 / #8: Chemical | ChemComp-PUY / | #9: Chemical | ChemComp-HEX / | #10: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.92 Å3/Da / Density % sol: 68.62 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 80 mM Bis-Tris, pH 6.0, 50 mM sodium citrate, 120 mM KCl, 10% PEG 4000, 0.5% N,N-dimethyldodecylamine N-oxide |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I24 / Wavelength: 0.9794 Å / Beamline: I24 / Wavelength: 0.9794 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Sep 1, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9794 Å / Relative weight: 1 |
| Reflection | Resolution: 3→50 Å / Num. obs: 94271 / % possible obs: 92.5 % / Redundancy: 3 % / Rmerge(I) obs: 0.175 / Net I/σ(I): 5.2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4DX5 Resolution: 3.2→34.928 Å / SU ML: 0.46 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 24.08
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.2→34.928 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






