+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 5k2h | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of GNNQQNY from yeast prion Sup35 in space group P212121 determined by MicroED | |||||||||||||||||||||
 要素 要素 | Eukaryotic peptide chain release factor GTP-binding subunit | |||||||||||||||||||||
 キーワード キーワード | PROTEIN FIBRIL / amyloid / yeast prion | |||||||||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Eukaryotic Translation Termination / translation release factor complex / translation release factor activity / nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / translational termination / cytoplasmic stress granule / regulation of translation / ribosome binding ...Eukaryotic Translation Termination / translation release factor complex / translation release factor activity / nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / translational termination / cytoplasmic stress granule / regulation of translation / ribosome binding / 加水分解酵素; 酸無水物に作用; GTPに作用・細胞または細胞小器官の運動に関与 / translation / GTPase activity / mRNA binding / GTP binding / identical protein binding / cytoplasm / cytosol 類似検索 - 分子機能 | |||||||||||||||||||||
| 生物種 |  | |||||||||||||||||||||
| 手法 | 電子線結晶学 / クライオ電子顕微鏡法 / 解像度: 1.05 Å | |||||||||||||||||||||
 データ登録者 データ登録者 | Rodriguez, J.A. / Sawaya, M.R. / Cascio, D. / Eisenberg, D.S. | |||||||||||||||||||||
| 資金援助 |  米国, 6件 米国, 6件
| |||||||||||||||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2016 ジャーナル: Proc Natl Acad Sci U S A / 年: 2016タイトル: Ab initio structure determination from prion nanocrystals at atomic resolution by MicroED. 著者: Michael R Sawaya / Jose Rodriguez / Duilio Cascio / Michael J Collazo / Dan Shi / Francis E Reyes / Johan Hattne / Tamir Gonen / David S Eisenberg /  要旨: Electrons, because of their strong interaction with matter, produce high-resolution diffraction patterns from tiny 3D crystals only a few hundred nanometers thick in a frozen-hydrated state. This ...Electrons, because of their strong interaction with matter, produce high-resolution diffraction patterns from tiny 3D crystals only a few hundred nanometers thick in a frozen-hydrated state. This discovery offers the prospect of facile structure determination of complex biological macromolecules, which cannot be coaxed to form crystals large enough for conventional crystallography or cannot easily be produced in sufficient quantities. Two potential obstacles stand in the way. The first is a phenomenon known as dynamical scattering, in which multiple scattering events scramble the recorded electron diffraction intensities so that they are no longer informative of the crystallized molecule. The second obstacle is the lack of a proven means of de novo phase determination, as is required if the molecule crystallized is insufficiently similar to one that has been previously determined. We show with four structures of the amyloid core of the Sup35 prion protein that, if the diffraction resolution is high enough, sufficiently accurate phases can be obtained by direct methods with the cryo-EM method microelectron diffraction (MicroED), just as in X-ray diffraction. The success of these four experiments dispels the concern that dynamical scattering is an obstacle to ab initio phasing by MicroED and suggests that structures of novel macromolecules can also be determined by direct methods. | |||||||||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  5k2h.cif.gz 5k2h.cif.gz | 14.3 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb5k2h.ent.gz pdb5k2h.ent.gz | 5.9 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  5k2h.json.gz 5k2h.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  5k2h_validation.pdf.gz 5k2h_validation.pdf.gz | 309.9 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  5k2h_full_validation.pdf.gz 5k2h_full_validation.pdf.gz | 309.5 KB | 表示 | |
| XML形式データ |  5k2h_validation.xml.gz 5k2h_validation.xml.gz | 1.3 KB | 表示 | |
| CIF形式データ |  5k2h_validation.cif.gz 5k2h_validation.cif.gz | 1.6 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/k2/5k2h https://data.pdbj.org/pub/pdb/validation_reports/k2/5k2h ftp://data.pdbj.org/pub/pdb/validation_reports/k2/5k2h ftp://data.pdbj.org/pub/pdb/validation_reports/k2/5k2h | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
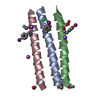
| 1 | 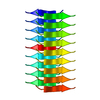
| ||||||||
| 単位格子 |
| ||||||||
| 詳細 | The biological unit is an extended pair of beta sheets comprising peptides at positions X,Y,Z and 1-X,1/2+Y,1/2-Z extended ad infinitum along the b crystal axis. |
- 要素
要素
| #1: タンパク質・ペプチド | 分子量: 836.807 Da / 分子数: 1 / 断片: UNP residues 7-13 / 由来タイプ: 合成 / 由来: (合成)  |
|---|---|
| #2: 水 | ChemComp-HOH / |
-実験情報
-実験
| 実験 | 手法: 電子線結晶学 |
|---|---|
| EM実験 | 試料の集合状態: 3D ARRAY / 3次元再構成法: 電子線結晶学 |
- 試料調製
試料調製
| 構成要素 | 名称: Prion fibril composed of a 7-residue segment of Sup35 タイプ: COMPLEX / Entity ID: #1 / 由来: MULTIPLE SOURCES |
|---|---|
| 分子量 | 値: 3.25 kDa/nm / 実験値: NO |
| 由来(天然) | 生物種:  |
| EM crystal formation | 装置: 24-well plate Atmosphere: in air, in sealed chamber, in equilibrium with reservoir solution 詳細: Grown in batch at ~20 degrees C in a microcentrifuge tube. Crystals grew within a day after seeding with NNQQNY-Zn crystals. Lipid mixture: none / 温度: 298 K / Time: 1 DAY |
| 緩衝液 | pH: 7 / 詳細: water |
| 試料 | 濃度: 10 mg/ml / 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES / 詳細: crystal |
| 試料支持 | グリッドの材料: COPPER / グリッドのサイズ: 300 divisions/in. / グリッドのタイプ: Quantifoil R2/2 |
| 急速凍結 | 装置: FEI VITROBOT MARK IV / 凍結剤: ETHANE / 詳細: Plunged into liquid ethane (FEI VITROBOT MARK IV) |
| 結晶化 | 温度: 273 K / 手法: バッチ法 / pH: 7 / 詳細: water |
-データ収集
| 実験機器 |  モデル: Tecnai F20 / 画像提供: FEI Company | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 顕微鏡 | モデル: FEI TECNAI F20 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 200 kV / 照射モード: FLOOD BEAM FIELD EMISSION GUN / 加速電圧: 200 kV / 照射モード: FLOOD BEAM | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 電子レンズ | モード: DIFFRACTION / アライメント法: BASIC | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 試料ホルダ | 凍結剤: NITROGEN 試料ホルダーモデル: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER 最高温度: 100 K / 最低温度: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 撮影 | 平均露光時間: 2 sec. / 電子線照射量: 0.01 e/Å2 フィルム・検出器のモデル: TVIPS TEMCAM-F416 (4k x 4k) Num. of diffraction images: 127 / 撮影したグリッド数: 2 詳細: The detector was operated in rolling shutter mode with 2x2 pixel binning. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 画像スキャン | サンプリングサイズ: 15.6 µm / 横: 4096 / 縦: 4096 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| EM回折 | カメラ長: 1350 mm | ||||||||||||||||||||||||||||||||||||||||||||||||||
| EM回折 シェル |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| EM回折 統計 | 詳細: Phase statistics are not applicable. No imaging was used. The phases were obtained by a crystallographic direct methods program, SHELXD. フーリエ空間範囲: 82.7 % / 再高解像度: 1 Å / 測定した強度の数: 16753 / 構造因子数: 2399 / 位相誤差: 0.1 ° / 位相残差: 0.1 ° / 位相誤差の除外基準: 0 / Rmerge: 15.1 / Rsym: 15.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 回折 | 平均測定温度: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 放射光源 | 由来: ELECTRON MICROSCOPE / タイプ: TECNAI F20 TEM / 波長: 0.0251 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 検出器 | タイプ: TVIPS F416 CMOS CAMERA / 検出器: CMOS / 日付: 2016年2月3日 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 放射波長 | 波長: 0.0251 Å / 相対比: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 反射 | 解像度: 1.05→20.11 Å / Num. obs: 1836 / % possible obs: 72.7 % / Observed criterion σ(I): -3 / Biso Wilson estimate: 8.654 Å2 / CC1/2: 0.992 / Rmerge(I) obs: 0.189 / Rrim(I) all: 0.218 / Χ2: 0.869 / Net I/σ(I): 4.18 / Num. measured all: 6795 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 反射 シェル | Diffraction-ID: 1 / Rejects: _
|
- 解析
解析
| ソフトウェア |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMソフトウェア |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EM 3D crystal entity | ∠α: 90 ° / ∠β: 90 ° / ∠γ: 90 ° / A: 23.16 Å / B: 4.93 Å / C: 40.51 Å / 空間群名: P212121 / 空間群番号: 19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF補正 | タイプ: NONE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3次元再構成 | 解像度の算出法: DIFFRACTION PATTERN/LAYERLINES 詳細: The density map was obtained using measured diffraction intensities and phases acquired from crystallographic direct methods program SHELXD. 対称性のタイプ: 3D CRYSTAL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子モデル構築 | B value: 4.7 / プロトコル: OTHER / 空間: RECIPROCAL / Target criteria: maximum likelihood | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化 | 解像度: 1.05→20.11 Å / Cor.coef. Fo:Fc: 0.966 / Cor.coef. Fo:Fc free: 0.979 / SU B: 0.613 / SU ML: 0.03 / SU R Cruickshank DPI: 0.0463 / 交差検証法: THROUGHOUT / σ(F): 0 / ESU R: 0.046 / ESU R Free: 0.044 / 詳細: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 溶媒の処理 | イオンプローブ半径: 0.8 Å / 減衰半径: 0.8 Å / VDWプローブ半径: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子変位パラメータ | Biso max: 18.76 Å2 / Biso mean: 4.705 Å2 / Biso min: 2.08 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: final / 解像度: 1.05→20.11 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS精密化 シェル | 解像度: 1.05→1.077 Å / Total num. of bins used: 20
|
 ムービー
ムービー コントローラー
コントローラー
















 PDBj
PDBj








