[English] 日本語
 Yorodumi
Yorodumi- PDB-5jlz: Crystal structure of HLA-DRB1*04:01 in complex with modified alph... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5jlz | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of HLA-DRB1*04:01 in complex with modified alpha-enolase peptide 26-40 with citrulline at the position 32 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | IMMUNE SYSTEM / HLA / autoimmune desease / rheumatoid arthritis / MHC class II / citrulline | |||||||||
| Function / homology |  Function and homology information Function and homology informationManipulation of host energy metabolism / positive regulation of plasminogen activation / regulation of interleukin-4 production / regulation of interleukin-10 production / phosphopyruvate hydratase / phosphopyruvate hydratase complex / phosphopyruvate hydratase activity / MHC class II receptor activity / negative regulation of hypoxia-induced intrinsic apoptotic signaling pathway / myeloid dendritic cell antigen processing and presentation ...Manipulation of host energy metabolism / positive regulation of plasminogen activation / regulation of interleukin-4 production / regulation of interleukin-10 production / phosphopyruvate hydratase / phosphopyruvate hydratase complex / phosphopyruvate hydratase activity / MHC class II receptor activity / negative regulation of hypoxia-induced intrinsic apoptotic signaling pathway / myeloid dendritic cell antigen processing and presentation / antigen processing and presentation of endogenous peptide antigen via MHC class II / autolysosome membrane / regulation of T-helper cell differentiation / positive regulation of CD4-positive, CD25-positive, alpha-beta regulatory T cell differentiation / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of muscle contraction / positive regulation of kinase activity / antigen processing and presentation of peptide or polysaccharide antigen via MHC class II / positive regulation of T cell mediated immune response to tumor cell / positive regulation of memory T cell differentiation / positive regulation of monocyte differentiation / inflammatory response to antigenic stimulus / CD4 receptor binding / Gluconeogenesis / M band / canonical glycolysis / Glycolysis / intermediate filament / T-helper 1 type immune response / transport vesicle membrane / Translocation of ZAP-70 to Immunological synapse / Phosphorylation of CD3 and TCR zeta chains / polysaccharide binding / negative regulation of type II interferon production / positive regulation of ATP biosynthetic process / humoral immune response / nuclear outer membrane / macrophage differentiation / Generation of second messenger molecules / immunological synapse / Co-inhibition by PD-1 / epidermis development / detection of bacterium / positive regulation of insulin secretion involved in cellular response to glucose stimulus / T cell receptor binding / negative regulation of T cell proliferation / MHC class II antigen presentation / transcription corepressor binding / trans-Golgi network membrane / glycolytic process / gluconeogenesis / RNA polymerase II transcription regulatory region sequence-specific DNA binding / lumenal side of endoplasmic reticulum membrane / protein tetramerization / negative regulation of inflammatory response to antigenic stimulus / clathrin-coated endocytic vesicle membrane / ER to Golgi transport vesicle membrane / negative regulation of cell growth / peptide antigen assembly with MHC class II protein complex / MHC class II protein complex / positive regulation of T cell mediated cytotoxicity / structural constituent of cytoskeleton / DNA-binding transcription repressor activity, RNA polymerase II-specific / response to virus / cognition / peptide antigen binding / antigen processing and presentation of exogenous peptide antigen via MHC class II / positive regulation of immune response / positive regulation of T cell activation / Interferon gamma signaling / positive regulation of protein phosphorylation / endocytic vesicle membrane / transcription corepressor activity / MHC class II protein complex binding / Downstream TCR signaling / T cell receptor signaling pathway / late endosome membrane / GTPase binding / early endosome membrane / cell cortex / adaptive immune response / positive regulation of viral entry into host cell / positive regulation of canonical NF-kappaB signal transduction / positive regulation of ERK1 and ERK2 cascade / lysosome / positive regulation of MAPK cascade / immune response / cadherin binding / Golgi membrane / external side of plasma membrane / lysosomal membrane / negative regulation of DNA-templated transcription / positive regulation of DNA-templated transcription / magnesium ion binding / cell surface / negative regulation of transcription by RNA polymerase II / signal transduction / protein homodimerization activity / extracellular space / RNA binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.99 Å MOLECULAR REPLACEMENT / Resolution: 1.99 Å | |||||||||
 Authors Authors | Dubnovitsky, A. / Kozhukh, G. / Sandalova, T. / Achour, A. | |||||||||
 Citation Citation |  Journal: Front Immunol / Year: 2016 Journal: Front Immunol / Year: 2016Title: Functional and Structural Characterization of a Novel HLA-DRB1*04:01-Restricted alpha-Enolase T Cell Epitope in Rheumatoid Arthritis. Authors: Gerstner, C. / Dubnovitsky, A. / Sandin, C. / Kozhukh, G. / Uchtenhagen, H. / James, E.A. / Ronnelid, J. / Ytterberg, A.J. / Pieper, J. / Reed, E. / Tandre, C. / Rieck, M. / Zubarev, R.A. / ...Authors: Gerstner, C. / Dubnovitsky, A. / Sandin, C. / Kozhukh, G. / Uchtenhagen, H. / James, E.A. / Ronnelid, J. / Ytterberg, A.J. / Pieper, J. / Reed, E. / Tandre, C. / Rieck, M. / Zubarev, R.A. / Ronnblom, L. / Sandalova, T. / Buckner, J.H. / Achour, A. / Malmstrom, V. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5jlz.cif.gz 5jlz.cif.gz | 333.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5jlz.ent.gz pdb5jlz.ent.gz | 272.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5jlz.json.gz 5jlz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jl/5jlz https://data.pdbj.org/pub/pdb/validation_reports/jl/5jlz ftp://data.pdbj.org/pub/pdb/validation_reports/jl/5jlz ftp://data.pdbj.org/pub/pdb/validation_reports/jl/5jlz | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5laxC  4mcyS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Refine code: _
NCS ensembles :
|
- Components
Components
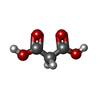
| #1: Protein | Mass: 21911.750 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: Extracellular Domain, UNP residues 26-206 / Source: (gene. exp.)  Homo sapiens (human) / Gene: HLA-DRA, HLA-DRA1 / Production host: Homo sapiens (human) / Gene: HLA-DRA, HLA-DRA1 / Production host:  #2: Protein | Mass: 23102.672 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: Extracellular Domain, UNP residues 30-219 / Source: (gene. exp.)  Homo sapiens (human) / Gene: HLA-DRB1 / Production host: Homo sapiens (human) / Gene: HLA-DRB1 / Production host:  #3: Protein/peptide | Mass: 1450.617 Da / Num. of mol.: 2 / Source method: obtained synthetically Details: Post-translational modification of Arg32 of human alpha-enolase Source: (synth.)  Homo sapiens (human) / References: UniProt: K7EM90, UniProt: P06733*PLUS Homo sapiens (human) / References: UniProt: K7EM90, UniProt: P06733*PLUS#4: Chemical | ChemComp-MLA / | #5: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.09 Å3/Da / Density % sol: 41.17 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 4 / Details: 0.1M sodium malonate, 15% PEG3350 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 0.976 Å / Beamline: ID23-1 / Wavelength: 0.976 Å |
| Detector | Type: DECTRIS PILATUS 2M / Detector: PIXEL / Date: Feb 6, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.976 Å / Relative weight: 1 |
| Reflection | Resolution: 1.99→47.76 Å / Num. obs: 51357 / % possible obs: 99.1 % / Redundancy: 5.3 % / Biso Wilson estimate: 38.3 Å2 / CC1/2: 0.994 / Net I/σ(I): 9.9 |
| Reflection shell | Resolution: 1.99→2.05 Å / Redundancy: 5.5 % / Mean I/σ(I) obs: 1.9 / % possible all: 96.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4MCY Resolution: 1.99→47.76 Å / Cor.coef. Fo:Fc: 0.956 / Cor.coef. Fo:Fc free: 0.929 / SU B: 14.329 / SU ML: 0.189 / Cross valid method: THROUGHOUT / ESU R: 0.228 / ESU R Free: 0.182 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45.751 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.99→47.76 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj