Entry Database : PDB / ID : 5j8hTitle Structure of calmodulin in a complex with a peptide derived from a calmodulin-dependent kinase Calmodulin Eukaryotic elongation factor 2 kinase Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / Authors Alphonse, S. / Lee, K. / Piserchio, A. / Tavares, C.D.J. / Giles, D.H. / Wellmann, R.M. / Dalby, K.N. / Ghose, R. Funding support Organization Grant number Country National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) GM084278 National Institutes of Health/National Institute on Minority Health and Health Disparities (NIH/NIMHD) G12 MD007603
Journal : Structure / Year : 2016Title : Structural Basis for the Recognition of Eukaryotic Elongation Factor 2 Kinase by Calmodulin.Authors : Lee, K. / Alphonse, S. / Piserchio, A. / Tavares, C.D. / Giles, D.H. / Wellmann, R.M. / Dalby, K.N. / Ghose, R. History Deposition Apr 7, 2016 Deposition site / Processing site Revision 1.0 Sep 7, 2016 Provider / Type Revision 1.1 Sep 21, 2016 Group Revision 1.2 Sep 20, 2017 Group / Database references / Structure summaryCategory / entity / pdbx_audit_supportItem / _entity.pdbx_number_of_molecules / _pdbx_audit_support.funding_organizationRevision 1.3 Dec 18, 2019 Group / Data collectionCategory / pdbx_nmr_software / pdbx_nmr_spectrometerItem / _pdbx_nmr_software.name / _pdbx_nmr_spectrometer.modelRevision 1.4 Jun 14, 2023 Group / Other / Category / pdbx_database_statusItem / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_nmr_dataRevision 1.5 May 15, 2024 Group / Database references / Category / chem_comp_bond / database_2 / Item
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
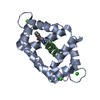
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) Authors
Authors United States, 2items
United States, 2items  Citation
Citation Journal: Structure / Year: 2016
Journal: Structure / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5j8h.cif.gz
5j8h.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5j8h.ent.gz
pdb5j8h.ent.gz PDB format
PDB format 5j8h.json.gz
5j8h.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/j8/5j8h
https://data.pdbj.org/pub/pdb/validation_reports/j8/5j8h ftp://data.pdbj.org/pub/pdb/validation_reports/j8/5j8h
ftp://data.pdbj.org/pub/pdb/validation_reports/j8/5j8h Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human)
Homo sapiens (human)
 Homo sapiens (human) / Gene: EEF2K / Plasmid: pET28b / Details (production host): pET28b-His-SUMO-CBD / Production host:
Homo sapiens (human) / Gene: EEF2K / Plasmid: pET28b / Details (production host): pET28b-His-SUMO-CBD / Production host: 
 Sample preparation
Sample preparation Processing
Processing Movie
Movie Controller
Controller











 PDBj
PDBj























 HNCA
HNCA