| Entry | Database: PDB / ID: 5f1a
|
|---|
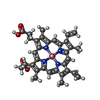
| Title | The Crystal Structure of Salicylate Bound to Human Cyclooxygenase-2 |
|---|
 Components Components | Prostaglandin G/H synthase 2 |
|---|
 Keywords Keywords | OXIDOREDUCTASE/INHIBITOR / Membrane Protein / Monotopic / Cyclooxygenase / Cyclooxygenase-2 / COX / PGHS / Salicylic acid / Salicylate / Inhibitor / Complex / OXIDOREDUCTASE-INHIBITOR complex |
|---|
| Function / homology |  Function and homology information Function and homology information
Biosynthesis of EPA-derived SPMs / Biosynthesis of DPAn-3 SPMs / positive regulation of platelet-derived growth factor production / Biosynthesis of DHA-derived SPMs / Biosynthesis of electrophilic ω-3 PUFA oxo-derivatives / oxidoreductase activity, acting on single donors with incorporation of molecular oxygen / Synthesis of 15-eicosatetraenoic acid derivatives / positive regulation of fibroblast growth factor production / cellular response to non-ionic osmotic stress / lipoxygenase pathway ...Biosynthesis of EPA-derived SPMs / Biosynthesis of DPAn-3 SPMs / positive regulation of platelet-derived growth factor production / Biosynthesis of DHA-derived SPMs / Biosynthesis of electrophilic ω-3 PUFA oxo-derivatives / oxidoreductase activity, acting on single donors with incorporation of molecular oxygen / Synthesis of 15-eicosatetraenoic acid derivatives / positive regulation of fibroblast growth factor production / cellular response to non-ionic osmotic stress / lipoxygenase pathway / positive regulation of transforming growth factor beta production / prostaglandin-endoperoxide synthase / prostaglandin-endoperoxide synthase activity / cyclooxygenase pathway / negative regulation of intrinsic apoptotic signaling pathway in response to osmotic stress / oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen / positive regulation of prostaglandin biosynthetic process / regulation of neuroinflammatory response / Synthesis of Prostaglandins (PG) and Thromboxanes (TX) / : / positive regulation of fever generation / response to selenium ion / prostaglandin secretion / cellular response to fluid shear stress / response to nematode / prostaglandin biosynthetic process / nuclear inner membrane / positive regulation of cell migration involved in sprouting angiogenesis / long-chain fatty acid biosynthetic process / Interleukin-10 signaling / decidualization / nuclear outer membrane / positive regulation of vascular endothelial growth factor production / positive regulation of brown fat cell differentiation / brown fat cell differentiation / embryo implantation / peroxidase activity / regulation of blood pressure / positive regulation of nitric oxide biosynthetic process / regulation of cell population proliferation / response to oxidative stress / regulation of inflammatory response / Interleukin-4 and Interleukin-13 signaling / cellular response to hypoxia / neuron projection / endoplasmic reticulum lumen / intracellular membrane-bounded organelle / heme binding / endoplasmic reticulum membrane / enzyme binding / endoplasmic reticulum / protein homodimerization activity / metal ion binding / cytoplasm / cytosolSimilarity search - Function : / Myeloperoxidase, subunit C / Haem peroxidase domain superfamily, animal type / Haem peroxidase, animal-type / Haem peroxidase domain superfamily, animal type / Animal haem peroxidase / Animal heme peroxidase superfamily profile. / Laminin / Laminin / EGF-like domain ...: / Myeloperoxidase, subunit C / Haem peroxidase domain superfamily, animal type / Haem peroxidase, animal-type / Haem peroxidase domain superfamily, animal type / Animal haem peroxidase / Animal heme peroxidase superfamily profile. / Laminin / Laminin / EGF-like domain / Haem peroxidase superfamily / EGF-like domain profile. / EGF-like domain / Ribbon / Orthogonal Bundle / Mainly Beta / Mainly AlphaSimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.38 Å molecular replacement / Resolution: 2.38 Å |
|---|
 Authors Authors | Lucido, M.J. / Orlando, B.J. / Malkowski, M.G. |
|---|
| Funding support |  United States, 1items United States, 1items | Organization | Grant number | Country |
|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | GM077176 |  United States United States |
|
|---|
 Citation Citation |  Journal: Biochemistry / Year: 2016 Journal: Biochemistry / Year: 2016
Title: Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight into the Formation of Products with Reversed Stereochemistry.
Authors: Lucido, M.J. / Orlando, B.J. / Vecchio, A.J. / Malkowski, M.G. |
|---|
| History | | Deposition | Nov 30, 2015 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Mar 16, 2016 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Sep 27, 2017 | Group: Author supporting evidence / Derived calculations / Refinement description
Category: pdbx_audit_support / pdbx_struct_oper_list / software
Item: _pdbx_audit_support.funding_organization / _pdbx_struct_oper_list.symmetry_operation / _software.classification |
|---|
| Revision 1.2 | Nov 20, 2019 | Group: Derived calculations / Category: pdbx_struct_conn_angle / struct_conn |
|---|
| Revision 1.3 | Dec 25, 2019 | Group: Author supporting evidence / Category: pdbx_audit_support / Item: _pdbx_audit_support.funding_organization |
|---|
| Revision 2.0 | Jul 29, 2020 | Group: Atomic model / Data collection ...Atomic model / Data collection / Derived calculations / Structure summary
Category: atom_site / atom_site_anisotrop ...atom_site / atom_site_anisotrop / chem_comp / entity / pdbx_branch_scheme / pdbx_chem_comp_identifier / pdbx_entity_branch / pdbx_entity_branch_descriptor / pdbx_entity_branch_link / pdbx_entity_branch_list / pdbx_entity_nonpoly / pdbx_nonpoly_scheme / pdbx_struct_assembly_gen / pdbx_struct_conn_angle / struct_asym / struct_conn / struct_site / struct_site_gen
Item: _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ..._atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.type_symbol / _atom_site_anisotrop.U[1][1] / _atom_site_anisotrop.U[1][2] / _atom_site_anisotrop.U[1][3] / _atom_site_anisotrop.U[2][2] / _atom_site_anisotrop.U[2][3] / _atom_site_anisotrop.U[3][3] / _atom_site_anisotrop.id / _atom_site_anisotrop.pdbx_auth_asym_id / _atom_site_anisotrop.pdbx_auth_atom_id / _atom_site_anisotrop.pdbx_auth_comp_id / _atom_site_anisotrop.pdbx_auth_seq_id / _atom_site_anisotrop.pdbx_label_asym_id / _atom_site_anisotrop.pdbx_label_atom_id / _atom_site_anisotrop.pdbx_label_comp_id / _atom_site_anisotrop.type_symbol / _chem_comp.mon_nstd_flag / _chem_comp.name / _chem_comp.type / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.pdbx_ptnr1_label_alt_id / _struct_conn.pdbx_role / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id
Description: Carbohydrate remediation / Provider: repository / Type: Remediation |
|---|
| Revision 2.1 | Nov 6, 2024 | Group: Data collection / Database references ...Data collection / Database references / Derived calculations / Structure summary
Category: chem_comp / chem_comp_atom ...chem_comp / chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature / pdbx_validate_chiral / struct_conn
Item: _chem_comp.pdbx_synonyms / _database_2.pdbx_DOI ..._chem_comp.pdbx_synonyms / _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_conn.pdbx_leaving_atom_flag |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.38 Å
molecular replacement / Resolution: 2.38 Å  Authors
Authors United States, 1items
United States, 1items  Citation
Citation Journal: Biochemistry / Year: 2016
Journal: Biochemistry / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5f1a.cif.gz
5f1a.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5f1a.ent.gz
pdb5f1a.ent.gz PDB format
PDB format 5f1a.json.gz
5f1a.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/f1/5f1a
https://data.pdbj.org/pub/pdb/validation_reports/f1/5f1a ftp://data.pdbj.org/pub/pdb/validation_reports/f1/5f1a
ftp://data.pdbj.org/pub/pdb/validation_reports/f1/5f1a Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: PTGS2, COX2 / Plasmid: pFastBac1 / Production host:
Homo sapiens (human) / Gene: PTGS2, COX2 / Plasmid: pFastBac1 / Production host: 









 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 17-ID / Wavelength: 1.033 Å
/ Beamline: 17-ID / Wavelength: 1.033 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.38→33.324 Å / SU ML: 0.24 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 21.9 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 2.38→33.324 Å / SU ML: 0.24 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 21.9 / Stereochemistry target values: ML Movie
Movie Controller
Controller
























 PDBj
PDBj











