[English] 日本語
 Yorodumi
Yorodumi- PDB-5ei9: Human PRDM9 allele-A ZnF Domain with Associated Recombination Hot... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ei9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human PRDM9 allele-A ZnF Domain with Associated Recombination Hotspot DNA Sequence I | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION/DNA / Protein-DNA complex / recombination / TRANSCRIPTION-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationrecombination hotspot binding / positive regulation of reciprocal meiotic recombination / male gamete generation / meiotic gene conversion / [histone H3]-lysine9 N-trimethyltransferase / positive regulation of fertilization / histone H4K20me methyltransferase activity / [histone H4]-N-methyl-L-lysine20 N-methyltransferase / histone H4K20 monomethyltransferase activity / histone H3K9 trimethyltransferase activity ...recombination hotspot binding / positive regulation of reciprocal meiotic recombination / male gamete generation / meiotic gene conversion / [histone H3]-lysine9 N-trimethyltransferase / positive regulation of fertilization / histone H4K20me methyltransferase activity / [histone H4]-N-methyl-L-lysine20 N-methyltransferase / histone H4K20 monomethyltransferase activity / histone H3K9 trimethyltransferase activity / [histone H4]-lysine20 N-methyltransferase / female gamete generation / [histone H3]-lysine4 N-trimethyltransferase / [histone H3]-lysine36 N-trimethyltransferase / histone H3K36 trimethyltransferase activity / double-strand break repair involved in meiotic recombination / homologous chromosome pairing at meiosis / histone H3K4 trimethyltransferase activity / histone H3K36 methyltransferase activity / histone H3K4 methyltransferase activity / Transferases; Transferring one-carbon groups; Methyltransferases / PKMTs methylate histone lysines / Meiotic recombination / chromosome / regulation of gene expression / methylation / transcription cis-regulatory region binding / regulation of DNA-templated transcription / negative regulation of apoptotic process / protein homodimerization activity / zinc ion binding / nucleoplasm / nucleus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.921 Å MOLECULAR REPLACEMENT / Resolution: 1.921 Å | ||||||
 Authors Authors | Patel, A. / Horton, J.R. / Wilson, G.G. / Zhang, X. / Cheng, X. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Genes Dev. / Year: 2016 Journal: Genes Dev. / Year: 2016Title: Structural basis for human PRDM9 action at recombination hot spots. Authors: Patel, A. / Horton, J.R. / Wilson, G.G. / Zhang, X. / Cheng, X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ei9.cif.gz 5ei9.cif.gz | 113.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ei9.ent.gz pdb5ei9.ent.gz | 81.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ei9.json.gz 5ei9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5ei9_validation.pdf.gz 5ei9_validation.pdf.gz | 471.3 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5ei9_full_validation.pdf.gz 5ei9_full_validation.pdf.gz | 479.7 KB | Display | |
| Data in XML |  5ei9_validation.xml.gz 5ei9_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  5ei9_validation.cif.gz 5ei9_validation.cif.gz | 22.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ei/5ei9 https://data.pdbj.org/pub/pdb/validation_reports/ei/5ei9 ftp://data.pdbj.org/pub/pdb/validation_reports/ei/5ei9 ftp://data.pdbj.org/pub/pdb/validation_reports/ei/5ei9 | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 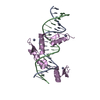
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: DNA chain | Mass: 6489.186 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)  Homo sapiens (human) Homo sapiens (human)#2: Protein | Mass: 16982.342 Da / Num. of mol.: 2 / Fragment: ZnF8-12 (UNP residues 717-858) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PRDM9, PFM6 / Plasmid: pGEX6p-1 / Production host: Homo sapiens (human) / Gene: PRDM9, PFM6 / Plasmid: pGEX6p-1 / Production host:  References: UniProt: Q9NQV7, histone-lysine N-methyltransferase #3: DNA chain | Mass: 6400.124 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)  Homo sapiens (human) Homo sapiens (human)#4: Chemical | ChemComp-ZN / #5: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.04 Å3/Da / Density % sol: 59.53 % |
|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, hanging drop / pH: 6 Details: 0.058 M Bistris Propane, 0.042 M Citric acid, 25% PEG3350 PH range: 6 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 1 Å / Beamline: 22-ID / Wavelength: 1 Å |
| Detector | Type: RAYONIX MX300-HS / Detector: CCD / Date: Jul 30, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.92→32.98 Å / Num. obs: 52634 / % possible obs: 99.9 % / Redundancy: 24.9 % / Rmerge(I) obs: 0.16 / Net I/σ(I): 16.39 |
| Reflection shell | Resolution: 1.92→2.02 Å / Redundancy: 13.8 % / Rmerge(I) obs: 0.948 / Mean I/σ(I) obs: 2.6 / % possible all: 99.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: Zn SAD determined structure Resolution: 1.921→32.98 Å / SU ML: 0.24 / Cross valid method: FREE R-VALUE / σ(F): 1.96 / Phase error: 31.12 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.921→32.98 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj










































