+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4z7p | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-ray structure of racemic ShK Q16K toxin | ||||||
 Components Components | Potassium channel toxin kappa-stichotoxin-She1a | ||||||
 Keywords Keywords | TOXIN / ShK toxin | ||||||
| Function / homology | ShKT domain / ShKT domain profile. / nematocyst / potassium channel regulator activity / toxin activity / defense response to bacterium / extracellular region / Kappa-stichotoxin-She3a Function and homology information Function and homology information | ||||||
| Biological species |  Stoichactis helianthus (sea anemone) Stoichactis helianthus (sea anemone) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.2 Å molecular replacement / Resolution: 1.2 Å | ||||||
 Authors Authors | Sickmier, E.A. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2015 Journal: J.Med.Chem. / Year: 2015Title: Pharmaceutical Optimization of Peptide Toxins for Ion Channel Targets: Potent, Selective, and Long-Lived Antagonists of Kv1.3. Authors: Murray, J.K. / Qian, Y.X. / Liu, B. / Elliott, R. / Aral, J. / Park, C. / Zhang, X. / Stenkilsson, M. / Salyers, K. / Rose, M. / Li, H. / Yu, S. / Andrews, K.L. / Colombero, A. / Werner, J. ...Authors: Murray, J.K. / Qian, Y.X. / Liu, B. / Elliott, R. / Aral, J. / Park, C. / Zhang, X. / Stenkilsson, M. / Salyers, K. / Rose, M. / Li, H. / Yu, S. / Andrews, K.L. / Colombero, A. / Werner, J. / Gaida, K. / Sickmier, E.A. / Miu, P. / Itano, A. / McGivern, J. / Gegg, C.V. / Sullivan, J.K. / Miranda, L.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4z7p.cif.gz 4z7p.cif.gz | 29.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4z7p.ent.gz pdb4z7p.ent.gz | 19.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4z7p.json.gz 4z7p.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4z7p_validation.pdf.gz 4z7p_validation.pdf.gz | 417 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4z7p_full_validation.pdf.gz 4z7p_full_validation.pdf.gz | 417 KB | Display | |
| Data in XML |  4z7p_validation.xml.gz 4z7p_validation.xml.gz | 3.8 KB | Display | |
| Data in CIF |  4z7p_validation.cif.gz 4z7p_validation.cif.gz | 4.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/z7/4z7p https://data.pdbj.org/pub/pdb/validation_reports/z7/4z7p ftp://data.pdbj.org/pub/pdb/validation_reports/z7/4z7p ftp://data.pdbj.org/pub/pdb/validation_reports/z7/4z7p | HTTPS FTP |
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 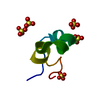
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein/peptide | Mass: 4070.943 Da / Num. of mol.: 1 / Mutation: Q16K / Source method: obtained synthetically / Source: (synth.)  Stoichactis helianthus (sea anemone) / References: UniProt: P29187 Stoichactis helianthus (sea anemone) / References: UniProt: P29187 | ||||
|---|---|---|---|---|---|
| #2: Chemical | ChemComp-SO4 / #3: Water | ChemComp-HOH / | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.9 Å3/Da / Density % sol: 35.38 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop Details: 0.1 M sodium acetate pH 4.5, 2-2.5 M ammonium sulfate |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1 Å / Beamline: 5.0.2 / Wavelength: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Mar 15, 2009 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.2→50 Å / Num. obs: 18716 / % possible obs: 99.4 % / Redundancy: 5.3 % / Rmerge(I) obs: 0.061 / Χ2: 1.013 / Net I/av σ(I): 22.419 / Net I/σ(I): 10 / Num. measured all: 98807 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.2→30.39 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.964 / WRfactor Rfree: 0.2443 / WRfactor Rwork: 0.2219 / FOM work R set: 0.8758 / SU B: 0.962 / SU ML: 0.019 / SU R Cruickshank DPI: 0.0399 / SU Rfree: 0.0401 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.04 / ESU R Free: 0.04 / Stereochemistry target values: MAXIMUM LIKELIHOOD MOLECULAR REPLACEMENT / Resolution: 1.2→30.39 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.964 / WRfactor Rfree: 0.2443 / WRfactor Rwork: 0.2219 / FOM work R set: 0.8758 / SU B: 0.962 / SU ML: 0.019 / SU R Cruickshank DPI: 0.0399 / SU Rfree: 0.0401 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.04 / ESU R Free: 0.04 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 58.25 Å2 / Biso mean: 16.774 Å2 / Biso min: 7.71 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.2→30.39 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.2→1.231 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller











 PDBj
PDBj


