+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 4rik | ||||||
|---|---|---|---|---|---|---|---|
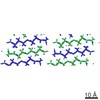
| タイトル | Amyloid forming segment, AVVTGVTAV, from the NAC domain of Parkinson's disease protein alpha-synuclein, residues 69-77 | ||||||
 要素 要素 | Alpha-synuclein | ||||||
 キーワード キーワード | LIPID BINDING PROTEIN / Amyloid / alpha-synuclein / Parkinson's Disease / Toxic Core / NAC | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber ...negative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / regulation of synaptic vesicle recycling / negative regulation of chaperone-mediated autophagy / mitochondrial membrane organization / regulation of reactive oxygen species biosynthetic process / negative regulation of platelet-derived growth factor receptor signaling pathway / positive regulation of protein localization to cell periphery / negative regulation of exocytosis / regulation of glutamate secretion / dopamine biosynthetic process / response to iron(II) ion / SNARE complex assembly / regulation of locomotion / positive regulation of neurotransmitter secretion / negative regulation of dopamine metabolic process / positive regulation of inositol phosphate biosynthetic process / regulation of macrophage activation / regulation of norepinephrine uptake / negative regulation of microtubule polymerization / synaptic vesicle transport / transporter regulator activity / synaptic vesicle priming / dopamine uptake involved in synaptic transmission / protein kinase inhibitor activity / mitochondrial ATP synthesis coupled electron transport / regulation of dopamine secretion / dynein complex binding / negative regulation of thrombin-activated receptor signaling pathway / positive regulation of receptor recycling / cuprous ion binding / nuclear outer membrane / response to magnesium ion / positive regulation of endocytosis / positive regulation of exocytosis / synaptic vesicle exocytosis / kinesin binding / synaptic vesicle endocytosis / enzyme inhibitor activity / cysteine-type endopeptidase inhibitor activity / negative regulation of serotonin uptake / response to type II interferon / regulation of presynapse assembly / alpha-tubulin binding / beta-tubulin binding / phospholipase binding / behavioral response to cocaine / supramolecular fiber organization / phospholipid metabolic process / cellular response to fibroblast growth factor stimulus / inclusion body / axon terminus / Hsp70 protein binding / cellular response to epinephrine stimulus / response to interleukin-1 / regulation of microtubule cytoskeleton organization / cellular response to copper ion / positive regulation of release of sequestered calcium ion into cytosol / adult locomotory behavior / SNARE binding / excitatory postsynaptic potential / protein tetramerization / phosphoprotein binding / microglial cell activation / ferrous iron binding / fatty acid metabolic process / regulation of long-term neuronal synaptic plasticity / synapse organization / protein destabilization / PKR-mediated signaling / phospholipid binding / receptor internalization / tau protein binding / long-term synaptic potentiation / terminal bouton / positive regulation of inflammatory response / synaptic vesicle membrane / actin cytoskeleton / actin binding / growth cone / cellular response to oxidative stress / neuron apoptotic process / cell cortex / response to lipopolysaccharide / histone binding / microtubule binding / molecular adaptor activity / chemical synaptic transmission / amyloid fibril formation / negative regulation of neuron apoptotic process / mitochondrial outer membrane / oxidoreductase activity 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  分子置換 / 解像度: 1.854 Å 分子置換 / 解像度: 1.854 Å | ||||||
 データ登録者 データ登録者 | Guenther, E.L. / Sawaya, M.R. / Ivanova, M. / Eisenberg, D.S. | ||||||
 引用 引用 |  ジャーナル: Nature / 年: 2015 ジャーナル: Nature / 年: 2015タイトル: Structure of the toxic core of α-synuclein from invisible crystals. 著者: Jose A Rodriguez / Magdalena I Ivanova / Michael R Sawaya / Duilio Cascio / Francis E Reyes / Dan Shi / Smriti Sangwan / Elizabeth L Guenther / Lisa M Johnson / Meng Zhang / Lin Jiang / Mark ...著者: Jose A Rodriguez / Magdalena I Ivanova / Michael R Sawaya / Duilio Cascio / Francis E Reyes / Dan Shi / Smriti Sangwan / Elizabeth L Guenther / Lisa M Johnson / Meng Zhang / Lin Jiang / Mark A Arbing / Brent L Nannenga / Johan Hattne / Julian Whitelegge / Aaron S Brewster / Marc Messerschmidt / Sébastien Boutet / Nicholas K Sauter / Tamir Gonen / David S Eisenberg /  要旨: The protein α-synuclein is the main component of Lewy bodies, the neuron-associated aggregates seen in Parkinson disease and other neurodegenerative pathologies. An 11-residue segment, which we term ...The protein α-synuclein is the main component of Lewy bodies, the neuron-associated aggregates seen in Parkinson disease and other neurodegenerative pathologies. An 11-residue segment, which we term NACore, appears to be responsible for amyloid formation and cytotoxicity of human α-synuclein. Here we describe crystals of NACore that have dimensions smaller than the wavelength of visible light and thus are invisible by optical microscopy. As the crystals are thousands of times too small for structure determination by synchrotron X-ray diffraction, we use micro-electron diffraction to determine the structure at atomic resolution. The 1.4 Å resolution structure demonstrates that this method can determine previously unknown protein structures and here yields, to our knowledge, the highest resolution achieved by any cryo-electron microscopy method to date. The structure exhibits protofibrils built of pairs of face-to-face β-sheets. X-ray fibre diffraction patterns show the similarity of NACore to toxic fibrils of full-length α-synuclein. The NACore structure, together with that of a second segment, inspires a model for most of the ordered portion of the toxic, full-length α-synuclein fibril, presenting opportunities for the design of inhibitors of α-synuclein fibrils. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  4rik.cif.gz 4rik.cif.gz | 9 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb4rik.ent.gz pdb4rik.ent.gz | 5.5 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  4rik.json.gz 4rik.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  4rik_validation.pdf.gz 4rik_validation.pdf.gz | 401.5 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  4rik_full_validation.pdf.gz 4rik_full_validation.pdf.gz | 401.4 KB | 表示 | |
| XML形式データ |  4rik_validation.xml.gz 4rik_validation.xml.gz | 2.4 KB | 表示 | |
| CIF形式データ |  4rik_validation.cif.gz 4rik_validation.cif.gz | 2.5 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/ri/4rik https://data.pdbj.org/pub/pdb/validation_reports/ri/4rik ftp://data.pdbj.org/pub/pdb/validation_reports/ri/4rik ftp://data.pdbj.org/pub/pdb/validation_reports/ri/4rik | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | x 10
| ||||||||
| 単位格子 |
| ||||||||
| 詳細 | The biological unit is a pair of beta-sheets. One sheet is composed of chain A and unit cell translations along the b dimension (for example, x,y+1,z, etc.). The other sheet is composed of the symmetry mate -x+1/2,y+1/2,-z+1, and its unit cell translations along b (for example, -x+1/2,y+3/2,-z+1, etc.). |
- 要素
要素
| #1: タンパク質・ペプチド | 分子量: 815.953 Da / 分子数: 1 / 由来タイプ: 合成 詳細: Synthetic peptide AVVTGVTAV corresponding to segment 69-77 of human alpha-synuclein 由来: (合成)  Homo sapiens (ヒト) / 参照: UniProt: P37840 Homo sapiens (ヒト) / 参照: UniProt: P37840 |
|---|---|
| #2: 水 | ChemComp-HOH / |
-実験情報
-実験
| 実験 | 手法:  X線回折 / 使用した結晶の数: 1 X線回折 / 使用した結晶の数: 1 |
|---|
- 試料調製
試料調製
| 結晶 | マシュー密度: 1.52 Å3/Da / 溶媒含有率: 19.22 % |
|---|---|
| 結晶化 | 温度: 273 K / 手法: 蒸気拡散法, ハンギングドロップ法 / pH: 4.6 詳細: 0.9M Ammonium Phosphate, 0.1M Sodium Acetate, pH 4.6, vapor diffusion, hanging drop, temperature 273K |
-データ収集
| 回折 | 平均測定温度: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 放射光源 | 由来:  シンクロトロン / サイト: シンクロトロン / サイト:  APS APS  / ビームライン: 24-ID-E / 波長: 0.9791 Å / ビームライン: 24-ID-E / 波長: 0.9791 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 検出器 | タイプ: ADSC QUANTUM 315 / 検出器: CCD / 日付: 2011年8月23日 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 放射 | プロトコル: SINGLE WAVELENGTH / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 放射波長 | 波長: 0.9791 Å / 相対比: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 反射 | 解像度: 1.85→100 Å / Num. all: 521 / Num. obs: 521 / % possible obs: 97.7 % / Observed criterion σ(I): -3 / 冗長度: 4.1 % / Rmerge(I) obs: 0.117 / Χ2: 0.961 / Net I/σ(I): 11.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 反射 シェル |
|
-位相決定
| 位相決定 | 手法:  分子置換 分子置換 |
|---|---|
| Phasing MR | Model details: Phaser MODE: MR_AUTO |
- 解析
解析
| ソフトウェア |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 構造決定の手法:  分子置換 分子置換開始モデル: poly alanine 解像度: 1.854→30 Å / Cor.coef. Fo:Fc: 0.978 / Cor.coef. Fo:Fc free: 0.961 / WRfactor Rfree: 0.236 / WRfactor Rwork: 0.19 / FOM work R set: 0.73 / SU B: 4.061 / SU ML: 0.112 / SU R Cruickshank DPI: 0.1735 / SU Rfree: 0.1526 / 交差検証法: THROUGHOUT / σ(F): 0 / ESU R: 0.173 / ESU R Free: 0.153 / 立体化学のターゲット値: MAXIMUM LIKELIHOOD 詳細: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 溶媒の処理 | イオンプローブ半径: 0.8 Å / 減衰半径: 0.8 Å / VDWプローブ半径: 1.2 Å / 溶媒モデル: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子変位パラメータ | Biso max: 53.1 Å2 / Biso mean: 17.341 Å2 / Biso min: 6.25 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: LAST / 解像度: 1.854→30 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS精密化 シェル | 解像度: 1.854→2.072 Å / Total num. of bins used: 5
|
 ムービー
ムービー コントローラー
コントローラー









 PDBj
PDBj

