[English] 日本語
 Yorodumi
Yorodumi- EMDB-3001: MicroED structure of the segment, GVVHGVTTVA, from the A53T famil... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3001 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | MicroED structure of the segment, GVVHGVTTVA, from the A53T familial mutant of Parkinson's disease protein, alpha-synuclein, residues 47-56 | |||||||||
 Map data Map data | 2mFo-dFc map covering multiple unit cells. Structure factors measured from micro electron diffraction (microED) methods. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Amyloid fibrils / alpha-synuclein / MicroED Crystallography / Parkinson's Disease / Peptide / familial mutation A53T | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber ...negative regulation of mitochondrial electron transport, NADH to ubiquinone / : / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / regulation of synaptic vesicle recycling / negative regulation of chaperone-mediated autophagy / mitochondrial membrane organization / regulation of reactive oxygen species biosynthetic process / positive regulation of protein localization to cell periphery / negative regulation of platelet-derived growth factor receptor signaling pathway / negative regulation of exocytosis / regulation of glutamate secretion / dopamine biosynthetic process / response to iron(II) ion / SNARE complex assembly / negative regulation of dopamine metabolic process / positive regulation of neurotransmitter secretion / regulation of macrophage activation / positive regulation of inositol phosphate biosynthetic process / regulation of norepinephrine uptake / regulation of locomotion / synaptic vesicle transport / negative regulation of microtubule polymerization / transporter regulator activity / synaptic vesicle priming / dopamine uptake involved in synaptic transmission / protein kinase inhibitor activity / regulation of dopamine secretion / negative regulation of thrombin-activated receptor signaling pathway / mitochondrial ATP synthesis coupled electron transport / positive regulation of receptor recycling / dynein complex binding / cuprous ion binding / nuclear outer membrane / response to magnesium ion / positive regulation of exocytosis / synaptic vesicle exocytosis / positive regulation of endocytosis / kinesin binding / synaptic vesicle endocytosis / enzyme inhibitor activity / cysteine-type endopeptidase inhibitor activity / response to type II interferon / negative regulation of serotonin uptake / regulation of presynapse assembly / alpha-tubulin binding / beta-tubulin binding / phospholipase binding / behavioral response to cocaine / supramolecular fiber organization / cellular response to fibroblast growth factor stimulus / phospholipid metabolic process / axon terminus / inclusion body / cellular response to epinephrine stimulus / Hsp70 protein binding / response to interleukin-1 / regulation of microtubule cytoskeleton organization / cellular response to copper ion / positive regulation of release of sequestered calcium ion into cytosol / SNARE binding / adult locomotory behavior / excitatory postsynaptic potential / protein tetramerization / phosphoprotein binding / microglial cell activation / ferrous iron binding / fatty acid metabolic process / regulation of long-term neuronal synaptic plasticity / synapse organization / PKR-mediated signaling / protein destabilization / phospholipid binding / receptor internalization / tau protein binding / long-term synaptic potentiation / terminal bouton / positive regulation of inflammatory response / synaptic vesicle membrane / actin cytoskeleton / actin binding / growth cone / cellular response to oxidative stress / neuron apoptotic process / cell cortex / histone binding / response to lipopolysaccharide / microtubule binding / chemical synaptic transmission / amyloid fibril formation / molecular adaptor activity / negative regulation of neuron apoptotic process / oxidoreductase activity / mitochondrial outer membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | electron crystallography / cryo EM / Resolution: 1.4 Å | |||||||||
 Authors Authors | Rodriguez JA / Ivanova M / Sawaya MR / Cascio D / Reyes F / Shi D / Johnson L / Guenther E / Sangwan S / Hattne J ...Rodriguez JA / Ivanova M / Sawaya MR / Cascio D / Reyes F / Shi D / Johnson L / Guenther E / Sangwan S / Hattne J / Nannenga B / Gonen T / Eisenberg D | |||||||||
 Citation Citation |  Journal: Nature / Year: 2015 Journal: Nature / Year: 2015Title: Structure of the toxic core of α-synuclein from invisible crystals. Authors: Jose A Rodriguez / Magdalena I Ivanova / Michael R Sawaya / Duilio Cascio / Francis E Reyes / Dan Shi / Smriti Sangwan / Elizabeth L Guenther / Lisa M Johnson / Meng Zhang / Lin Jiang / Mark ...Authors: Jose A Rodriguez / Magdalena I Ivanova / Michael R Sawaya / Duilio Cascio / Francis E Reyes / Dan Shi / Smriti Sangwan / Elizabeth L Guenther / Lisa M Johnson / Meng Zhang / Lin Jiang / Mark A Arbing / Brent L Nannenga / Johan Hattne / Julian Whitelegge / Aaron S Brewster / Marc Messerschmidt / Sébastien Boutet / Nicholas K Sauter / Tamir Gonen / David S Eisenberg /  Abstract: The protein α-synuclein is the main component of Lewy bodies, the neuron-associated aggregates seen in Parkinson disease and other neurodegenerative pathologies. An 11-residue segment, which we term ...The protein α-synuclein is the main component of Lewy bodies, the neuron-associated aggregates seen in Parkinson disease and other neurodegenerative pathologies. An 11-residue segment, which we term NACore, appears to be responsible for amyloid formation and cytotoxicity of human α-synuclein. Here we describe crystals of NACore that have dimensions smaller than the wavelength of visible light and thus are invisible by optical microscopy. As the crystals are thousands of times too small for structure determination by synchrotron X-ray diffraction, we use micro-electron diffraction to determine the structure at atomic resolution. The 1.4 Å resolution structure demonstrates that this method can determine previously unknown protein structures and here yields, to our knowledge, the highest resolution achieved by any cryo-electron microscopy method to date. The structure exhibits protofibrils built of pairs of face-to-face β-sheets. X-ray fibre diffraction patterns show the similarity of NACore to toxic fibrils of full-length α-synuclein. The NACore structure, together with that of a second segment, inspires a model for most of the ordered portion of the toxic, full-length α-synuclein fibril, presenting opportunities for the design of inhibitors of α-synuclein fibrils. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3001.map.gz emd_3001.map.gz | 263.8 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3001-v30.xml emd-3001-v30.xml emd-3001.xml emd-3001.xml | 12.2 KB 12.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3001.png emd_3001.png | 399 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3001 http://ftp.pdbj.org/pub/emdb/structures/EMD-3001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3001 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3001 | HTTPS FTP |
-Related structure data
| Related structure data |  4znnMC 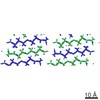 3028C  4rikC  4rilC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3001.map.gz / Format: CCP4 / Size: 300.8 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3001.map.gz / Format: CCP4 / Size: 300.8 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 2mFo-dFc map covering multiple unit cells. Structure factors measured from micro electron diffraction (microED) methods. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X: 0.44825 Å / Y: 0.3925 Å / Z: 0.45875 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : GVVHGVTTVA, a segment from the A53T familial mutant of Parkinson'...
| Entire | Name: GVVHGVTTVA, a segment from the A53T familial mutant of Parkinson's disease protein, alpha-synuclein, residues 47-56 |
|---|---|
| Components |
|
-Supramolecule #1000: GVVHGVTTVA, a segment from the A53T familial mutant of Parkinson'...
| Supramolecule | Name: GVVHGVTTVA, a segment from the A53T familial mutant of Parkinson's disease protein, alpha-synuclein, residues 47-56 type: sample / ID: 1000 / Details: crystalline fibrils / Oligomeric state: crystalline fibrils / Number unique components: 1 |
|---|
-Macromolecule #1: alpha synuclein residues 47-56
| Macromolecule | Name: alpha synuclein residues 47-56 / type: protein_or_peptide / ID: 1 / Name.synonym: a-syn Details: alpha synuclein residues 47-56 with A53T mutation. Synthesized chemically. Number of copies: 1 / Oligomeric state: fibril / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: Human Homo sapiens (human) / synonym: Human |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron crystallography |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 7 / Details: 50 mM phosphate, 0.1% w/v DMSO |
| Grid | Details: quantifoil holey-carbon EM grid, 300 mesh copper |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 100 K / Instrument: FEI VITROBOT MARK IV Method: Nanocrystals were deposited onto a quantifoil holey-carbon EM grid in a 2-3 microliter drop after appropriate dilution, which optimized for crystal density on the grid. All grids were then ...Method: Nanocrystals were deposited onto a quantifoil holey-carbon EM grid in a 2-3 microliter drop after appropriate dilution, which optimized for crystal density on the grid. All grids were then blotted and vitrified by plunging into liquid ethane using a Vitrobot Mark IV (FEI), then transferring to liquid nitrogen for storage. |
| Details | Crystals grew in batch. In a microcentrifuge tube at 37 degrees C with shaking. |
| Crystal formation | Details: Crystals grew in batch. In a microcentrifuge tube at 37 degrees C with shaking. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Min: 99 K / Max: 101 K / Average: 100 K |
| Details | very low dose data collection. Spot size 11. |
| Date | Apr 20, 2015 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Number real images: 343 / Average electron dose: 0.10000000000000001 e/Å2 / Camera length: 2230 / Details: Diffraction images are available upon request. / Bits/pixel: 16 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: DIFFRACTION |
| Sample stage | Specimen holder: liquid nitrogen cooled / Specimen holder model: GATAN LIQUID NITROGEN / Tilt angle min: -66 / Tilt angle max: 66 / Tilt series - Axis1 - Min angle: -66 ° / Tilt series - Axis1 - Max angle: 66 ° |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Diffraction images were processed with XDS and XSCALE. Please note that the unit cell length B is 4.71 A. This value was not accepted as valid on the web submission page. |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 1.4 Å / Resolution method: DIFFRACTION PATTERN/LAYERLINES Details: The diffraction data set contains intensities measured from three crystals. |
| Crystal parameters | Unit cell - A: 17.930 Å / Unit cell - B: 4.71 Å / Unit cell - C: 33.030 Å / Unit cell - γ: 90.0 ° / Unit cell - α: 90 ° / Unit cell - β: 94.33 ° / Space group: P 1 21 1 |
 Movie
Movie Controller
Controller




 Y (Sec.)
Y (Sec.) X (Row.)
X (Row.) Z (Col.)
Z (Col.)





















