[English] 日本語
 Yorodumi
Yorodumi- PDB-4msj: Crystal structure of S. pombe AMSH-like protease SST2 catalytic d... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4msj | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of S. pombe AMSH-like protease SST2 catalytic domain from P212121 space group | ||||||
 Components Components | AMSH-like protease sst2 | ||||||
 Keywords Keywords | HYDROLASE / helix-beta-helix sandwich / ubiquitin / deubiquitination / zinc metalloprotease / lysine 63-linked polyubiquitin / cytosol | ||||||
| Function / homology |  Function and homology information Function and homology informationMetalloprotease DUBs / cytoplasm to vacuole targeting by the NVT pathway / late endosome to vacuole transport via multivesicular body sorting pathway / protein transport to vacuole involved in ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway / Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases / late endosome to vacuole transport / protein K63-linked deubiquitination / metal-dependent deubiquitinase activity / K63-linked polyubiquitin modification-dependent protein binding / K63-linked deubiquitinase activity ...Metalloprotease DUBs / cytoplasm to vacuole targeting by the NVT pathway / late endosome to vacuole transport via multivesicular body sorting pathway / protein transport to vacuole involved in ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway / Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases / late endosome to vacuole transport / protein K63-linked deubiquitination / metal-dependent deubiquitinase activity / K63-linked polyubiquitin modification-dependent protein binding / K63-linked deubiquitinase activity / cell division site / ubiquitin binding / endosome / zinc ion binding / membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Shrestha, R.K. / Ronau, J.A. / Das, C. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2014 Journal: Biochemistry / Year: 2014Title: Insights into the Mechanism of Deubiquitination by JAMM Deubiquitinases from Cocrystal Structures of the Enzyme with the Substrate and Product. Authors: Shrestha, R.K. / Ronau, J.A. / Davies, C.W. / Guenette, R.G. / Strieter, E.R. / Paul, L.N. / Das, C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4msj.cif.gz 4msj.cif.gz | 130.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4msj.ent.gz pdb4msj.ent.gz | 101.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4msj.json.gz 4msj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ms/4msj https://data.pdbj.org/pub/pdb/validation_reports/ms/4msj ftp://data.pdbj.org/pub/pdb/validation_reports/ms/4msj ftp://data.pdbj.org/pub/pdb/validation_reports/ms/4msj | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4jxeSC  4k1rC 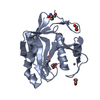 4ms7C  4msdC  4msmC  4msqC  4nqlC  4pqtC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 21981.732 Da / Num. of mol.: 3 / Fragment: catalytic domain, UNP residues 245-435 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Strain: 972/ATCC 24843 / Gene: sst2, SPAC19B12.10 / Plasmid: pGEX-6P-1 / Production host:  References: UniProt: Q9P371, Hydrolases; Acting on peptide bonds (peptidases); Omega peptidases |
|---|
-Non-polymers , 5 types, 236 molecules 








| #2: Chemical | ChemComp-EDO / #3: Chemical | #4: Chemical | ChemComp-GLY / | #5: Chemical | ChemComp-ZN / #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.26 Å3/Da / Density % sol: 45.62 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8 Details: 0.2 M ammonium phosphate dibasic, 20% w/v PEG 3350, 1.0 M glycine, pH 7.6, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 23-ID-D / Wavelength: 1.033 Å / Beamline: 23-ID-D / Wavelength: 1.033 Å |
| Detector | Type: MAR scanner 300 mm plate / Detector: IMAGE PLATE / Date: Oct 7, 2012 |
| Radiation | Monochromator: Si 111 Channel / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.033 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→50 Å / Num. all: 56615 / Num. obs: 55256 / % possible obs: 97.9 % / Observed criterion σ(F): 2.9 / Observed criterion σ(I): 2.9 / Redundancy: 6 % / Rmerge(I) obs: 0.087 / Rsym value: 0.087 / Net I/σ(I): 17.8 |
| Reflection shell | Resolution: 1.8→1.83 Å / Redundancy: 6.1 % / Rmerge(I) obs: 0.628 / Mean I/σ(I) obs: 2.9 / Rsym value: 0.628 / % possible all: 96.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB CODE 4JXE Resolution: 1.8→47.31 Å / SU ML: 0.19 / σ(F): 1.37 / Phase error: 23.66 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→47.31 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj








