[English] 日本語
 Yorodumi
Yorodumi- PDB-4gy9: Crystal Structure of Medicago truncatula Nodulin 13 (MtN13) in co... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4gy9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Medicago truncatula Nodulin 13 (MtN13) in complex with N6-isopentenyladenine (2iP) | ||||||
 Components Components | MtN13 protein | ||||||
 Keywords Keywords | PLANT PROTEIN / PR-10 FOLD / nodulin / nodulation / legume plant-rhizobium symbiosis / cytokinin binding | ||||||
| Function / homology |  Function and homology information Function and homology informationnodulation / cytokinin-activated signaling pathway / abscisic acid binding / abscisic acid-activated signaling pathway / protein phosphatase inhibitor activity / defense response / signaling receptor activity Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.04 Å molecular replacement / Resolution: 2.04 Å | ||||||
 Authors Authors | Ruszkowski, M. / Sikorski, M. / Jaskolski, M. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2013 Journal: Acta Crystallogr.,Sect.D / Year: 2013Title: The landscape of cytokinin binding by a plant nodulin. Authors: Ruszkowski, M. / Szpotkowski, K. / Sikorski, M. / Jaskolski, M. #1:  Journal: J.Mol.Biol. / Year: 2008 Journal: J.Mol.Biol. / Year: 2008Title: Lupinus luteus pathogenesis-related protein as a reservoir for cytokinin. Authors: Fernandes, H. / Pasternak, O. / Bujacz, G. / Bujacz, A. / Sikorski, M.M. / Jaskolski, M. #2:  Journal: Febs J. / Year: 2009 Journal: Febs J. / Year: 2009Title: Cytokinin-induced structural adaptability of a Lupinus luteus PR-10 protein. Authors: Fernandes, H. / Bujacz, A. / Bujacz, G. / Jelen, F. / Jasinski, M. / Kachlicki, P. / Otlewski, J. / Sikorski, M.M. / Jaskolski, M. #3:  Journal: Plant Cell / Year: 2006 Journal: Plant Cell / Year: 2006Title: Crystal structure of Vigna radiata cytokinin-specific binding protein in complex with zeatin. Authors: Pasternak, O. / Bujacz, G.D. / Fujimoto, Y. / Hashimoto, Y. / Jelen, F. / Otlewski, J. / Sikorski, M.M. / Jaskolski, M. #4:  Journal: J.Struct.Biol. / Year: 2010 Journal: J.Struct.Biol. / Year: 2010Title: Crystal structure of Hyp-1, a St. John's wort protein implicated in the biosynthesis of hypericin. Authors: Michalska, K. / Fernandes, H. / Sikorski, M. / Jaskolski, M. #5: Journal: Mol.Plant Microbe Interact. / Year: 1996 Title: Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Authors: Gamas, P. / Niebel Fde, C. / Lescure, N. / Cullimore, J. #6: Journal: Mol.Plant Microbe Interact. / Year: 1998 Title: Symbiosis-specific expression of two Medicago truncatula nodulin genes, MtN1 and MtN13, encoding products homologous to plant defense proteins. Authors: Gamas, P. / de Billy, F. / Truchet, G. #7:  Journal: J.Mol.Biol. / Year: 2002 Journal: J.Mol.Biol. / Year: 2002Title: Crystal structures of two homologous pathogenesis-related proteins from yellow lupine. Authors: Biesiadka, J. / Bujacz, G. / Sikorski, M.M. / Jaskolski, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4gy9.cif.gz 4gy9.cif.gz | 86.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4gy9.ent.gz pdb4gy9.ent.gz | 64 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4gy9.json.gz 4gy9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gy/4gy9 https://data.pdbj.org/pub/pdb/validation_reports/gy/4gy9 ftp://data.pdbj.org/pub/pdb/validation_reports/gy/4gy9 ftp://data.pdbj.org/pub/pdb/validation_reports/gy/4gy9 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4jhgC  4jhhC  4jhiC  3rws C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| 2 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 18773.020 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   | ||||
|---|---|---|---|---|---|
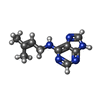
| #2: Chemical | ChemComp-ZIP / | ||||
| #3: Chemical | ChemComp-NA / #4: Chemical | ChemComp-MLI / | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.02 Å3/Da / Density % sol: 69.39 % |
|---|---|
| Crystal grow | Temperature: 292 K / pH: 7.1 Details: 1.5 M sodium malonate, Dehydration at elevated sodium malonate concentration (1.9 M), pH 7.1, VAPOR DIFFUSION, HANGING DROP, temperature 292K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  PETRA III, EMBL c/o DESY PETRA III, EMBL c/o DESY  / Beamline: P14 (MX2) / Wavelength: 1.22343 / Beamline: P14 (MX2) / Wavelength: 1.22343 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RAYONIX MX-225 / Detector: CCD / Date: May 31, 2012 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: SAGITALLY FOCUSED SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.22343 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Number: 287179 / Rmerge(I) obs: 0.099 / D res high: 2.036 Å / Num. obs: 20324 / % possible obs: 99.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diffraction reflection shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.04→50 Å / Num. obs: 20324 / % possible obs: 99.7 % / Observed criterion σ(I): -3 / Redundancy: 14.1 % / Biso Wilson estimate: 33.3 Å2 / Rmerge(I) obs: 0.099 / Net I/σ(I): 24.33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Resolution: 2.04→2.16 Å / Rmerge(I) obs: 0.706 / Mean I/σ(I) obs: 3.95 / % possible all: 98.2 |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3RWS  3rws Resolution: 2.04→48.3 Å / Occupancy max: 1 / Occupancy min: 0.37 / SU ML: 0.16 / Phase error: 18.33 / Stereochemistry target values: ENGH & HUBER / Details: HYDROGEN ATOMS WERE ADDED AT RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 36.05 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.04→48.3 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller



















 PDBj
PDBj