[English] 日本語
 Yorodumi
Yorodumi- PDB-4jhg: Crystal Structure of Medicago truncatula Nodulin 13 (MtN13) in co... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4jhg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Medicago truncatula Nodulin 13 (MtN13) in complex with trans-zeatin | |||||||||
 Components Components | MtN13 protein | |||||||||
 Keywords Keywords | PLANT PROTEIN / PR-10 FOLD / nodulin / nodulation / legume-bacteria symbiosis / nitrogen fixation / CYTOKININ BINDING | |||||||||
| Function / homology |  Function and homology information Function and homology informationnodulation / cytokinin-activated signaling pathway / abscisic acid binding / abscisic acid-activated signaling pathway / protein phosphatase inhibitor activity / defense response / signaling receptor activity Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.85 Å MOLECULAR REPLACEMENT / Resolution: 1.85 Å | |||||||||
 Authors Authors | Ruszkowski, M. / Tusnio, K. / Ciesielska, A. / Brzezinski, K. / Dauter, M. / Dauter, Z. / Sikorski, M. / Jaskolski, M. | |||||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2013 Journal: Acta Crystallogr.,Sect.D / Year: 2013Title: The landscape of cytokinin binding by a plant nodulin. Authors: Ruszkowski, M. / Szpotkowski, K. / Sikorski, M. / Jaskolski, M. #1: Journal: Febs J. / Year: 2013 Title: Structural and functional aspects of PR-10 proteins. Authors: Fernandes, H. / Michalska, K. / Sikorski, M. / Jaskolski, M. #2:  Journal: J.Mol.Biol. / Year: 2008 Journal: J.Mol.Biol. / Year: 2008Title: Lupinus luteus pathogenesis-related protein as a reservoir for cytokinin. Authors: Fernandes, H. / Pasternak, O. / Bujacz, G. / Bujacz, A. / Sikorski, M.M. / Jaskolski, M. #3:  Journal: Febs J. / Year: 2009 Journal: Febs J. / Year: 2009Title: Cytokinin-induced structural adaptability of a Lupinus luteus PR-10 protein. Authors: Fernandes, H. / Bujacz, A. / Bujacz, G. / Jelen, F. / Jasinski, M. / Kachlicki, P. / Otlewski, J. / Sikorski, M.M. / Jaskolski, M. #4:  Journal: Plant Cell / Year: 2006 Journal: Plant Cell / Year: 2006Title: Crystal structure of Vigna radiata cytokinin-specific binding protein in complex with zeatin. Authors: Pasternak, O. / Bujacz, G.D. / Fujimoto, Y. / Hashimoto, Y. / Jelen, F. / Otlewski, J. / Sikorski, M.M. / Jaskolski, M. #5: Journal: Mol.Plant Microbe Interact. / Year: 1998 Title: Symbiosis-specific expression of two Medicago truncatula nodulin genes, MtN1 and MtN13, encoding products homologous to plant defense proteins. Authors: Gamas, P. / de Billy, F. / Truchet, G. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4jhg.cif.gz 4jhg.cif.gz | 87.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4jhg.ent.gz pdb4jhg.ent.gz | 64.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4jhg.json.gz 4jhg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jh/4jhg https://data.pdbj.org/pub/pdb/validation_reports/jh/4jhg ftp://data.pdbj.org/pub/pdb/validation_reports/jh/4jhg ftp://data.pdbj.org/pub/pdb/validation_reports/jh/4jhg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4gy9C  4jhhC  4jhiC  2qimS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 18773.020 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   | ||
|---|---|---|---|
| #2: Chemical | ChemComp-ZEA / ( | ||
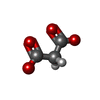
| #3: Chemical | ChemComp-MLI / | ||
| #4: Chemical | | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.02 Å3/Da / Density % sol: 69.41 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 8 Details: 1.9 M SODIUM MALONATE, 200 mM NaCl, 50 mM Tris-HCl, protein was incubated overnight with trans-zeatin prior to crystallization, pH 8.0, VAPOR DIFFUSION, HANGING DROP, temperature 292K |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-BM / Wavelength: 1 Å / Beamline: 22-BM / Wavelength: 1 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Mar 12, 2011 / Details: FOCUSING MIRRORS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: SAGITALLY FOCUSED SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.85→30 Å / Num. all: 26897 / Num. obs: 26831 / % possible obs: 99.9 % / Observed criterion σ(I): -3 / Redundancy: 10.5 % / Rmerge(I) obs: 0.075 / Χ2: 1.092 / Net I/σ(I): 9.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 2qim Resolution: 1.85→24.92 Å / Occupancy max: 1 / Occupancy min: 0.33 / SU ML: 0.16 / Cross valid method: R-free / Phase error: 21.92 / Stereochemistry target values: Engh & Huber / Details: Hydrogen atoms were added at riding positions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 122.28 Å2 / Biso mean: 47.6 Å2 / Biso min: 20.67 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→24.92 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 10
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj