[English] 日本語
 Yorodumi
Yorodumi- PDB-4bqy: HIF prolyl hydroxylase 2 (PHD2/ EGLN1) in complex with Fe(II) and... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4bqy | ||||||
|---|---|---|---|---|---|---|---|
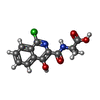
| Title | HIF prolyl hydroxylase 2 (PHD2/ EGLN1) in complex with Fe(II) and N-[(1-chloro-4-hydroxyisoquinolin-3-yl)carbonyl]alanine | ||||||
 Components Components | EGL NINE HOMOLOG 1 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / 2-OXOGLUTARATE / DIOXYGENASE / EGLN / OXYGENASE / HYPOXIA / DNA-BINDING / METAL-BINDING / TRANSCRIPTION / DSBH / FACIAL TRIAD / ASPARAGINYL/ ASPARTYL HYDROXYLASE / TRANSCRIPTION AND EPIGENETIC REGULATION / SIGNALING / DEVELOPMENT / CELL STRUCTURE / ANKYRIN REPEAT DOMAIN / ARD / BETA-HYDROXYLATION / TRANSCRIPTION ACTIVATOR/INHIBITOR / PHOSPHORYLATION / S-NITROSYLATION | ||||||
| Function / homology |  Function and homology information Function and homology informationpeptidyl-proline 4-dioxygenase activity / hypoxia-inducible factor-proline dioxygenase activity / hypoxia-inducible factor-proline dioxygenase / peptidyl-proline dioxygenase activity / negative regulation of cyclic-nucleotide phosphodiesterase activity / intracellular oxygen homeostasis / labyrinthine layer development / regulation protein catabolic process at postsynapse / 2-oxoglutarate-dependent dioxygenase activity / heart trabecula formation ...peptidyl-proline 4-dioxygenase activity / hypoxia-inducible factor-proline dioxygenase activity / hypoxia-inducible factor-proline dioxygenase / peptidyl-proline dioxygenase activity / negative regulation of cyclic-nucleotide phosphodiesterase activity / intracellular oxygen homeostasis / labyrinthine layer development / regulation protein catabolic process at postsynapse / 2-oxoglutarate-dependent dioxygenase activity / heart trabecula formation / regulation of modification of postsynaptic structure / cardiac muscle tissue morphogenesis / L-ascorbic acid binding / response to nitric oxide / ventricular septum morphogenesis / regulation of angiogenesis / regulation of neuron apoptotic process / negative regulation of DNA-binding transcription factor activity / ferrous iron binding / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / cellular response to hypoxia / intracellular iron ion homeostasis / response to hypoxia / postsynaptic density / intracellular membrane-bounded organelle / glutamatergic synapse / enzyme binding / positive regulation of transcription by RNA polymerase II / zinc ion binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.53 Å MOLECULAR REPLACEMENT / Resolution: 1.53 Å | ||||||
 Authors Authors | Chowdhury, R. / McDonough, M.A. / Yeoh, K.K. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: ACS Chem. Biol. / Year: 2013 Journal: ACS Chem. Biol. / Year: 2013Title: Selective small molecule probes for the hypoxia inducible factor (HIF) prolyl hydroxylases. Authors: Chowdhury, R. / Candela-Lena, J.I. / Chan, M.C. / Greenald, D.J. / Yeoh, K.K. / Tian, Y.M. / McDonough, M.A. / Tumber, A. / Rose, N.R. / Conejo-Garcia, A. / Demetriades, M. / Mathavan, S. / ...Authors: Chowdhury, R. / Candela-Lena, J.I. / Chan, M.C. / Greenald, D.J. / Yeoh, K.K. / Tian, Y.M. / McDonough, M.A. / Tumber, A. / Rose, N.R. / Conejo-Garcia, A. / Demetriades, M. / Mathavan, S. / Kawamura, A. / Lee, M.K. / van Eeden, F. / Pugh, C.W. / Ratcliffe, P.J. / Schofield, C.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4bqy.cif.gz 4bqy.cif.gz | 100.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4bqy.ent.gz pdb4bqy.ent.gz | 78.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4bqy.json.gz 4bqy.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bq/4bqy https://data.pdbj.org/pub/pdb/validation_reports/bq/4bqy ftp://data.pdbj.org/pub/pdb/validation_reports/bq/4bqy ftp://data.pdbj.org/pub/pdb/validation_reports/bq/4bqy | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4bqwC  4bqxC  2g19S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 28096.941 Da / Num. of mol.: 1 / Fragment: CATALYTIC DOMAIN, RESIDUES 181-426 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Production host: HOMO SAPIENS (human) / Production host:  References: UniProt: Q9GZT9, Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor |
|---|---|
| #2: Chemical | ChemComp-FE2 / |
| #3: Chemical | ChemComp-FNT / ( |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.6 Å3/Da / Density % sol: 52.3 % / Description: NONE |
|---|---|
| Crystal grow | pH: 6.5 Details: 0.1M MES PH6.5, 1.6M AMMONIUM SULFATE, 3% DIOXANE, 0.002M FECL2, NEAR ANAEROBIC |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM14 / Wavelength: 0.979 / Beamline: BM14 / Wavelength: 0.979 |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Jul 11, 2009 / Details: MIRRORS |
| Radiation | Monochromator: GRAPHITE / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979 Å / Relative weight: 1 |
| Reflection | Resolution: 1.55→36.3 Å / Num. obs: 43534 / % possible obs: 95.5 % / Observed criterion σ(I): 2 / Redundancy: 2.5 % / Biso Wilson estimate: 21.53 Å2 / Rmerge(I) obs: 0.05 / Net I/σ(I): 16.14 |
| Reflection shell | Resolution: 1.55→1.62 Å / Redundancy: 2.3 % / Rmerge(I) obs: 0.44 / Mean I/σ(I) obs: 1.8 / % possible all: 94.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2G19 Resolution: 1.53→36.296 Å / SU ML: 0.17 / σ(F): 1.34 / Phase error: 24.79 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 48.3911 Å2 / ksol: 0.350989 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 28.2 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.53→36.296 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj