[English] 日本語
 Yorodumi
Yorodumi- PDB-4afm: Structural and biochemical characterization of a novel Carbohydra... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4afm | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structural and biochemical characterization of a novel Carbohydrate Binding Module of endoglucanase Cel5A from Eubacterium cellulosolvens. | ||||||
 Components Components | ENDOGLUCANASE CEL5A | ||||||
 Keywords Keywords | HYDROLASE / CARBOHYDRATE BINDING MODULE / FAMILY 5 GLYCOSIDE HYDROLASE | ||||||
| Function / homology |  Function and homology information Function and homology informationbeta-glucosidase activity / cellulose catabolic process / cell surface / extracellular region Similarity search - Function | ||||||
| Biological species |  EUBACTERIUM CELLULOSOLVENS (bacteria) EUBACTERIUM CELLULOSOLVENS (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.25 Å MOLECULAR REPLACEMENT / Resolution: 1.25 Å | ||||||
 Authors Authors | Luis, A.S. / Venditto, I. / Prates, J.A.M. / Ferreira, L.M.A. / Gilbert, H.J. / Fontes, C.M.G.A. / Najmudin, S. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2013 Journal: J.Biol.Chem. / Year: 2013Title: Understanding How Non-Catalytic Carbohydrate Binding Modules Can Display Specificity for Xyloglucan. Authors: Luis, A.S. / Venditto, I. / Prates, J.A.M. / Ferrieira, L.M.A. / Temple, M.J. / Rogowski, A. / Basle, A. / Xue, J. / Knox, J.P. / Najmudin, S. / Fontes, C.M.G.A. / Gilbert, H.J. #1: Journal: Acta Crystallogr.,Sect.F / Year: 2011 Title: Overproduction, Purification, Crystallization and Preliminary X-Ray Characterization of a Novel Carbohydrate-Binding Module of Endoglucanase Cel5A from Eubacterium Cellulosolvens. Authors: Luis, A.S. / Alves, V.D. / Romao, M.J. / Prates, J.A.M. / Fontes, C.M.G.A. / Najmudin, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4afm.cif.gz 4afm.cif.gz | 72 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4afm.ent.gz pdb4afm.ent.gz | 54.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4afm.json.gz 4afm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4afm_validation.pdf.gz 4afm_validation.pdf.gz | 456 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4afm_full_validation.pdf.gz 4afm_full_validation.pdf.gz | 459.1 KB | Display | |
| Data in XML |  4afm_validation.xml.gz 4afm_validation.xml.gz | 9.8 KB | Display | |
| Data in CIF |  4afm_validation.cif.gz 4afm_validation.cif.gz | 13.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/af/4afm https://data.pdbj.org/pub/pdb/validation_reports/af/4afm ftp://data.pdbj.org/pub/pdb/validation_reports/af/4afm ftp://data.pdbj.org/pub/pdb/validation_reports/af/4afm | HTTPS FTP |
-Related structure data
| Related structure data |  2ypjC  4aekSC 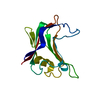 4aemC  4afdC  4ba6C C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 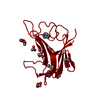
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 14879.080 Da / Num. of mol.: 1 / Fragment: CARBOHYDRATE BINDING MODULE, RESIDUES 37-170 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  EUBACTERIUM CELLULOSOLVENS (bacteria) / Production host: EUBACTERIUM CELLULOSOLVENS (bacteria) / Production host:  | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | ChemComp-ACT / #4: Water | ChemComp-HOH / | Has protein modification | Y | Nonpolymer details | GLYCEROL (GOL): FROM THE CRYOPROTEC | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.65 Å3/Da / Density % sol: 66 % / Description: NONE |
|---|---|
| Crystal grow | Temperature: 292 K / pH: 4.6 Details: 80 MG/ML OF PROTEIN AT 292 K WERE GROWN IN 0.2 M AMMONIUM SULFATE, 0.1 M SODIUM ACETATE TRIHYDRATE PH 4.6, 26% W/V PEG 2K MME. CRYSTALS WERE SOAKED WITH 10 MM CELLOHEXAOSE FOR A FEW HOURS. ...Details: 80 MG/ML OF PROTEIN AT 292 K WERE GROWN IN 0.2 M AMMONIUM SULFATE, 0.1 M SODIUM ACETATE TRIHYDRATE PH 4.6, 26% W/V PEG 2K MME. CRYSTALS WERE SOAKED WITH 10 MM CELLOHEXAOSE FOR A FEW HOURS. 30% GLYCEROL WAS USED AS A CRYOPROTECTANT. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.97934 / Beamline: PROXIMA 1 / Wavelength: 0.97934 |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Apr 4, 2011 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97934 Å / Relative weight: 1 |
| Reflection | Resolution: 1.25→38.74 Å / Num. obs: 35557 / % possible obs: 91.2 % / Redundancy: 21.6 % / Rmerge(I) obs: 0.07 / Net I/σ(I): 28.2 |
| Reflection shell | Resolution: 1.25→1.32 Å / Redundancy: 6.2 % / Rmerge(I) obs: 0.4 / Mean I/σ(I) obs: 4 / % possible all: 62.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4AEK Resolution: 1.25→38.7 Å / Cor.coef. Fo:Fc: 0.967 / Cor.coef. Fo:Fc free: 0.963 / SU B: 1.074 / SU ML: 0.023 / Cross valid method: THROUGHOUT / ESU R: 0.043 / ESU R Free: 0.044 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT U VALUES.PHENIX-1.7.2-869 WAS USED IN THE PENULTIMATE STEP FOR OCCUPANCY REFINEMENT AND ...Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT U VALUES.PHENIX-1.7.2-869 WAS USED IN THE PENULTIMATE STEP FOR OCCUPANCY REFINEMENT AND TLS GROUP DETERMINATION. RESIDUES 37-38 AND 166-170 ARE DISORDERED. ASP A118 WAS MODELLED WITH ALTERNATE CONFORMATION. IT MAY ALSO HAVE RADIATION DAMAGE AS IN PDB 4AFD. DISORDERED SIDE CHAINS WERE MODELED STEREOCHEMICALLY. FOLLOWING WERE GIVEN ALTERNATE CONFORMATIONS, SER A45, GLU A51, GLN A67, GLN A71, MSE A73, GLU A82, ILE A83, IL3 A95 GLU A100, SER A103, SER A112, VAL A116, GLN A126, LYS A130, MSE A154, VAL A160 AND SER A162.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 14.031 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.25→38.7 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj



