+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3zgo | ||||||
|---|---|---|---|---|---|---|---|
| Title | Re-refined structure of the human Sirt2 apoform | ||||||
 Components Components | NAD-DEPENDENT PROTEIN DEACETYLASE SIRTUIN-2 | ||||||
 Keywords Keywords | HYDROLASE / NAD+-DEPENDENT DEACETYLASE / SIRTUIN | ||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to caloric restriction / negative regulation of oligodendrocyte progenitor proliferation / negative regulation of striated muscle tissue development / negative regulation of satellite cell differentiation / histone H4K16 deacetylase activity, NAD-dependent / positive regulation of attachment of spindle microtubules to kinetochore / positive regulation of meiotic nuclear division / NAD-dependent protein demyristoylase activity / NAD-dependent protein depalmitoylase activity / paranodal junction ...cellular response to caloric restriction / negative regulation of oligodendrocyte progenitor proliferation / negative regulation of striated muscle tissue development / negative regulation of satellite cell differentiation / histone H4K16 deacetylase activity, NAD-dependent / positive regulation of attachment of spindle microtubules to kinetochore / positive regulation of meiotic nuclear division / NAD-dependent protein demyristoylase activity / NAD-dependent protein depalmitoylase activity / paranodal junction / tubulin deacetylation / peptidyl-lysine deacetylation / lateral loop / NLRP3 inflammasome complex assembly / mitotic nuclear membrane reassembly / tubulin deacetylase activity / negative regulation of NLRP3 inflammasome complex assembly / paranode region of axon / regulation of exit from mitosis / Schmidt-Lanterman incisure / negative regulation of peptidyl-threonine phosphorylation / positive regulation of fatty acid biosynthetic process / NAD-dependent protein lysine deacetylase activity / regulation of phosphorylation / protein acetyllysine N-acetyltransferase / myelination in peripheral nervous system / rDNA heterochromatin formation / protein deacetylation / histone deacetylase activity, NAD-dependent / positive regulation of oocyte maturation / juxtaparanode region of axon / Initiation of Nuclear Envelope (NE) Reformation / chromatin silencing complex / meiotic spindle / protein lysine deacetylase activity / histone deacetylase activity / regulation of myelination / response to redox state / positive regulation of DNA binding / histone acetyltransferase binding / negative regulation of fat cell differentiation / negative regulation of reactive oxygen species metabolic process / positive regulation of cell division / NAD+ poly-ADP-ribosyltransferase activity / NAD+ binding / glial cell projection / positive regulation of execution phase of apoptosis / subtelomeric heterochromatin formation / heterochromatin / lipid catabolic process / cellular response to epinephrine stimulus / centriole / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / substantia nigra development / negative regulation of autophagy / epigenetic regulation of gene expression / ubiquitin binding / meiotic cell cycle / negative regulation of protein catabolic process / autophagy / histone deacetylase binding / spindle / mitotic spindle / heterochromatin formation / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / myelin sheath / chromosome / growth cone / cellular response to oxidative stress / midbody / cellular response to hypoxia / perikaryon / DNA-binding transcription factor binding / microtubule / proteasome-mediated ubiquitin-dependent protein catabolic process / chromosome, telomeric region / regulation of cell cycle / innate immune response / cell division / negative regulation of DNA-templated transcription / chromatin binding / centrosome / nucleolus / perinuclear region of cytoplasm / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / mitochondrion / zinc ion binding / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / OTHER / Resolution: 1.63 Å SYNCHROTRON / OTHER / Resolution: 1.63 Å | ||||||
 Authors Authors | Moniot, S. / Steegborn, C. | ||||||
 Citation Citation |  Journal: Nat.Struct.Biol. / Year: 2001 Journal: Nat.Struct.Biol. / Year: 2001Title: Structure of the Histone Deacetylase Sirt2. Authors: Finnin, M.S. / Donigian, J.R. / Pavletich, N.P. | ||||||
| History |
| ||||||
| Remark 0 | THIS ENTRY 3ZGO REFLECTS AN ALTERNATIVE MODELING OF THE ORIGINAL STRUCTURAL DATA (R1J8FSF) ...THIS ENTRY 3ZGO REFLECTS AN ALTERNATIVE MODELING OF THE ORIGINAL STRUCTURAL DATA (R1J8FSF) DETERMINED BY AUTHORS OF THE PDB ENTRY 1J8F: N.P.PAVLETICH,M.S.FINNIN,J.R.DONIGIAN |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3zgo.cif.gz 3zgo.cif.gz | 421.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3zgo.ent.gz pdb3zgo.ent.gz | 348.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3zgo.json.gz 3zgo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zg/3zgo https://data.pdbj.org/pub/pdb/validation_reports/zg/3zgo ftp://data.pdbj.org/pub/pdb/validation_reports/zg/3zgo ftp://data.pdbj.org/pub/pdb/validation_reports/zg/3zgo | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 36660.125 Da / Num. of mol.: 3 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PGEX-4T3 / Production host: HOMO SAPIENS (human) / Plasmid: PGEX-4T3 / Production host:  References: UniProt: Q8IXJ6, Hydrolases; Acting on carbon-nitrogen bonds, other than peptide bonds; In linear amides |
|---|
-Non-polymers , 6 types, 1095 molecules 



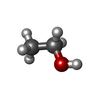






| #2: Chemical | | #3: Chemical | ChemComp-P6G / | #4: Chemical | ChemComp-PGE / #5: Chemical | ChemComp-EDO / | #6: Chemical | ChemComp-EOH / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.33 Å3/Da / Density % sol: 47.24 % / Description: AUTHOR USED THE SF DATA FROM ENTRY 1J8F. |
|---|
-Data collection
| Diffraction |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| |||||||||||||||
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE | |||||||||||||||
| Radiation |
| |||||||||||||||
| Radiation wavelength |
| |||||||||||||||
| Reflection | Observed criterion σ(I): 0 |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.7.0032 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: OTHER / Resolution: 1.63→19.84 Å / Cor.coef. Fo:Fc: 0.958 / Cor.coef. Fo:Fc free: 0.945 / SU B: 3.113 / SU ML: 0.056 / Cross valid method: THROUGHOUT / ESU R: 0.093 / ESU R Free: 0.09 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES WITH TLS ADDED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 22.055 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.63→19.84 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj












