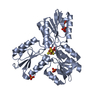
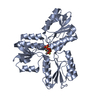
Entry Database : PDB / ID : 3zglTitle Crystal structures of Escherichia coli IspH in complex with AMBPP a potent inhibitor of the methylerythritol phosphate pathway 4-HYDROXY-3-METHYLBUT-2-ENYL DIPHOSPHATE REDUCTASE Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / Biological species ESCHERICHIA COLI (E. coli)Method / / / Resolution : 1.68 Å Authors Borel, F. / Barbier, E. / Kratsutsky, S. / Janthawornpong, K. / Rohmer, M. / Dale Poulter, C. / Ferrer, J.L. / Seemann, M. Journal : Chembiochem / Year : 2017Title : Further Insight into Crystal Structures of Escherichia coli IspH/LytB in Complex with Two Potent Inhibitors of the MEP Pathway: A Starting Point for Rational Design of New Antimicrobials.Authors : Borel, F. / Barbier, E. / Krasutsky, S. / Janthawornpong, K. / Chaignon, P. / Poulter, C.D. / Ferrer, J.L. / Seemann, M. History Deposition Dec 18, 2012 Deposition site / Processing site Revision 1.0 Jan 9, 2013 Provider / Type Revision 1.1 Nov 29, 2017 Group / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _citation_author.name Revision 1.2 Dec 20, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Other / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_database_status / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_sf / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.68 Å
MOLECULAR REPLACEMENT / Resolution: 1.68 Å  Authors
Authors Citation
Citation Journal: Chembiochem / Year: 2017
Journal: Chembiochem / Year: 2017 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3zgl.cif.gz
3zgl.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3zgl.ent.gz
pdb3zgl.ent.gz PDB format
PDB format 3zgl.json.gz
3zgl.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/zg/3zgl
https://data.pdbj.org/pub/pdb/validation_reports/zg/3zgl ftp://data.pdbj.org/pub/pdb/validation_reports/zg/3zgl
ftp://data.pdbj.org/pub/pdb/validation_reports/zg/3zgl

 Links
Links Assembly
Assembly


 Components
Components

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID23-1 / Wavelength: 0.979
/ Beamline: ID23-1 / Wavelength: 0.979  Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller




















 PDBj
PDBj



