[English] 日本語
 Yorodumi
Yorodumi- PDB-3kv5: Structure of KIAA1718, human Jumonji demethylase, in complex with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3kv5 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of KIAA1718, human Jumonji demethylase, in complex with N-oxalylglycine | ||||||
 Components Components | JmjC domain-containing histone demethylation protein 1D | ||||||
 Keywords Keywords | H3K4me3 binding protein / Transferase / Epigenetics / Histone Code / Jumonji lysine demethylase / Metal-binding / Zinc / Zinc-finger | ||||||
| Function / homology |  Function and homology information Function and homology informationhistone H3K9me/H3K9me2 demethylase activity / histone H3K27me2/H3K27me3 demethylase activity / histone H4K20 demethylase activity / histone H3K36 demethylase activity / [histone H3]-dimethyl-L-lysine9 demethylase / 2-oxoglutarate-dependent dioxygenase activity / midbrain development / histone H3K9 demethylase activity / histone demethylase activity / transcription coregulator activity ...histone H3K9me/H3K9me2 demethylase activity / histone H3K27me2/H3K27me3 demethylase activity / histone H4K20 demethylase activity / histone H3K36 demethylase activity / [histone H3]-dimethyl-L-lysine9 demethylase / 2-oxoglutarate-dependent dioxygenase activity / midbrain development / histone H3K9 demethylase activity / histone demethylase activity / transcription coregulator activity / HDMs demethylate histones / Signaling by BRAF and RAF1 fusions / chromatin remodeling / iron ion binding / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / nucleolus / zinc ion binding / nucleoplasm / nucleus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.39 Å MOLECULAR REPLACEMENT / Resolution: 2.39 Å | ||||||
 Authors Authors | Horton, J.R. / Upadhyay, A.K. / Qi, H.H. / Zhang, X. / Shi, Y. / Cheng, X. | ||||||
 Citation Citation |  Journal: Nat.Struct.Mol.Biol. / Year: 2010 Journal: Nat.Struct.Mol.Biol. / Year: 2010Title: Enzymatic and structural insights for substrate specificity of a family of jumonji histone lysine demethylases. Authors: Horton, J.R. / Upadhyay, A.K. / Qi, H.H. / Zhang, X. / Shi, Y. / Cheng, X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3kv5.cif.gz 3kv5.cif.gz | 199.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3kv5.ent.gz pdb3kv5.ent.gz | 154.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3kv5.json.gz 3kv5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kv/3kv5 https://data.pdbj.org/pub/pdb/validation_reports/kv/3kv5 ftp://data.pdbj.org/pub/pdb/validation_reports/kv/3kv5 ftp://data.pdbj.org/pub/pdb/validation_reports/kv/3kv5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3kv4C  3kv6C  3kv9C  3kvaC  3kvbC  1wepS  2yu2S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules DA
| #1: Protein | Mass: 55317.512 Da / Num. of mol.: 2 / Fragment: Residues 1-488 Source method: isolated from a genetically manipulated source Details: GST-fusion / Source: (gene. exp.)  Homo sapiens (human) / Gene: JHDM1D, KIAA1718 / Plasmid: pXC720 / Production host: Homo sapiens (human) / Gene: JHDM1D, KIAA1718 / Plasmid: pXC720 / Production host:  |
|---|
-Non-polymers , 5 types, 338 molecules 


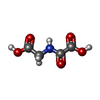





| #2: Chemical | ChemComp-ZN / #3: Chemical | #4: Chemical | #5: Chemical | ChemComp-OGA / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.67 Å3/Da / Density % sol: 66.46 % |
|---|---|
| Crystal grow | pH: 6 Details: 5-10% (v/v) polyethylene glycol 3350, 0.2 M KSCN, and 0.1 M BisTris pH 6.0, VAPOR DIFFUSION, HANGING DROP |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 1 / Beamline: 22-ID / Wavelength: 1 |
| Detector | Detector: CCD / Date: Mar 17, 2009 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.39→34.82 Å / Num. obs: 62010 / % possible obs: 94.8 % / Observed criterion σ(I): 0 / Redundancy: 8 % / Biso Wilson estimate: 21.1 Å2 / Rmerge(I) obs: 0.055 / Net I/σ(I): 9.7 |
| Reflection shell | Resolution: 2.39→2.48 Å / Redundancy: 4.2 % / Rmerge(I) obs: 0.637 / Mean I/σ(I) obs: 1.7 / % possible all: 81.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRIES 2YU2 AND 1WEP Resolution: 2.39→34.82 Å / Rfactor Rfree error: 0.005 / Data cutoff high absF: 289052.96 / Data cutoff low absF: 0 / Isotropic thermal model: OVERALL ANISOTROPIC B VALUE / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: ENGH & HUBER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 41.6772 Å2 / ksol: 0.35 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 41.6 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.39→34.82 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.39→2.48 Å / Rfactor Rfree error: 0.022 / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj








