+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3e6p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human meizothrombin desF1 | |||||||||
 Components Components | (Prothrombin) x 2 | |||||||||
 Keywords Keywords | HYDROLASE / Thrombin / meizothrombin / allostery / linkage / Na+ binding / Acute phase / Blood coagulation / Cleavage on pair of basic residues / Disease mutation / Gamma-carboxyglutamic acid / Glycoprotein / Kringle / Protease / Secreted / Serine protease / Zymogen | |||||||||
| Function / homology |  Function and homology information Function and homology information: / thrombospondin receptor activity / Defective factor XII causes hereditary angioedema / thrombin / thrombin-activated receptor signaling pathway / negative regulation of astrocyte differentiation / regulation of blood coagulation / neutrophil-mediated killing of gram-negative bacterium / positive regulation of phospholipase C-activating G protein-coupled receptor signaling pathway / Defective F8 cleavage by thrombin ...: / thrombospondin receptor activity / Defective factor XII causes hereditary angioedema / thrombin / thrombin-activated receptor signaling pathway / negative regulation of astrocyte differentiation / regulation of blood coagulation / neutrophil-mediated killing of gram-negative bacterium / positive regulation of phospholipase C-activating G protein-coupled receptor signaling pathway / Defective F8 cleavage by thrombin / Platelet Aggregation (Plug Formation) / ligand-gated ion channel signaling pathway / positive regulation of collagen biosynthetic process / blood coagulation, fibrin clot formation / negative regulation of platelet activation / negative regulation of blood coagulation / negative regulation of fibrinolysis / positive regulation of blood coagulation / regulation of cytosolic calcium ion concentration / Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus / Gamma-carboxylation of protein precursors / Common Pathway of Fibrin Clot Formation / Removal of aminoterminal propeptides from gamma-carboxylated proteins / fibrinolysis / Intrinsic Pathway of Fibrin Clot Formation / negative regulation of proteolysis / negative regulation of cytokine production involved in inflammatory response / Regulation of Complement cascade / Peptide ligand-binding receptors / positive regulation of release of sequestered calcium ion into cytosol / acute-phase response / Cell surface interactions at the vascular wall / positive regulation of receptor signaling pathway via JAK-STAT / growth factor activity / lipopolysaccharide binding / positive regulation of insulin secretion / platelet activation / positive regulation of protein localization to nucleus / response to wounding / Golgi lumen / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / positive regulation of reactive oxygen species metabolic process / blood coagulation / antimicrobial humoral immune response mediated by antimicrobial peptide / regulation of cell shape / heparin binding / Thrombin signalling through proteinase activated receptors (PARs) / positive regulation of cell growth / blood microparticle / G alpha (q) signalling events / cell surface receptor signaling pathway / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / endoplasmic reticulum lumen / receptor ligand activity / signaling receptor binding / serine-type endopeptidase activity / positive regulation of cell population proliferation / calcium ion binding / proteolysis / extracellular space / extracellular exosome / extracellular region / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | |||||||||
 Authors Authors | Papaconstantinou, M.E. / Gandhi, P. / Chen, Z. / Bah, A. / Di Cera, E. | |||||||||
 Citation Citation |  Journal: Cell.Mol.Life Sci. / Year: 2008 Journal: Cell.Mol.Life Sci. / Year: 2008Title: Na(+) binding to meizothrombin desF1. Authors: Papaconstantinou, M.E. / Gandhi, P.S. / Chen, Z. / Bah, A. / Di Cera, E. #1:  Journal: Structure / Year: 1997 Journal: Structure / Year: 1997Title: New insights into the regulation of the blood clotting cascade derived from the X-ray crystal structure of bovine meizothrombin des F1 in complex with PPACK. Authors: Martin, P.D. / Malkowski, M.G. / Box, J. / Esmon, C.T. / Edwards, B.F. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3e6p.cif.gz 3e6p.cif.gz | 103.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3e6p.ent.gz pdb3e6p.ent.gz | 76.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3e6p.json.gz 3e6p.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e6/3e6p https://data.pdbj.org/pub/pdb/validation_reports/e6/3e6p ftp://data.pdbj.org/pub/pdb/validation_reports/e6/3e6p ftp://data.pdbj.org/pub/pdb/validation_reports/e6/3e6p | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 2 molecules LH
| #1: Protein | Mass: 17586.156 Da / Num. of mol.: 1 Fragment: Activation peptide fragment 2 and thrombin light chain: UNP residues 206-363 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: F2 / Plasmid: HPC4-pNUT / Cell (production host): baby hamster kidney cells / Production host: Homo sapiens (human) / Gene: F2 / Plasmid: HPC4-pNUT / Cell (production host): baby hamster kidney cells / Production host:  |
|---|---|
| #2: Protein | Mass: 29780.219 Da / Num. of mol.: 1 / Fragment: Thrombin heavy chain: UNP residues 364-622 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: F2 / Plasmid: HPC4-pNUT / Cell (production host): baby hamster kidney cells / Production host: Homo sapiens (human) / Gene: F2 / Plasmid: HPC4-pNUT / Cell (production host): baby hamster kidney cells / Production host:  |
-Sugars , 1 types, 1 molecules
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 4 types, 211 molecules 
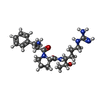





| #4: Chemical | ChemComp-SO4 / |
|---|---|
| #5: Chemical | ChemComp-DFK / |
| #6: Chemical | ChemComp-NA / |
| #7: Water | ChemComp-HOH / |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.89 Å3/Da / Density % sol: 68.39 % |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 6.6 Details: 0.2M Na2(SO4), 23% PEG 3350, pH 6.6, VAPOR DIFFUSION, HANGING DROP, temperature 295K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 14-BM-C / Wavelength: 0.9 Å / Beamline: 14-BM-C / Wavelength: 0.9 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jun 28, 2008 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→40 Å / Num. all: 43798 / Num. obs: 40469 / % possible obs: 92.4 % / Observed criterion σ(I): -0.5 / Redundancy: 11.8 % / Rmerge(I) obs: 0.112 / Net I/σ(I): 17.7 |
| Reflection shell | Resolution: 2.1→2.18 Å / Redundancy: 5.2 % / Rmerge(I) obs: 0.438 / Mean I/σ(I) obs: 2 / Num. unique all: 3398 / % possible all: 79 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entries 1A0H, 1SHH Resolution: 2.1→32.58 Å / Cor.coef. Fo:Fc: 0.951 / Cor.coef. Fo:Fc free: 0.931 / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: 1. HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. 2. Residues L251-L286 were modeled using bovine meizothrombin (PDB ID code 1A0H) and assigned an occupancy of zero due to lack of ...Details: 1. HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. 2. Residues L251-L286 were modeled using bovine meizothrombin (PDB ID code 1A0H) and assigned an occupancy of zero due to lack of electron density. 3. Residues with missing atoms listed in Remark 470 are due to lack of electron density for side chains and were modeled as alanines.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 44.5 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→32.58 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.1→2.16 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller















 PDBj
PDBj











