[English] 日本語
 Yorodumi
Yorodumi- PDB-3dpf: Crystal structure of the complex between MMP-8 and a non-zinc che... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3dpf | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the complex between MMP-8 and a non-zinc chelating inhibitor | ||||||
 Components Components | Neutrophil collagenase | ||||||
 Keywords Keywords | HYDROLASE / selective inhibition / non-zinc chelating inhibitors / Calcium / Collagen degradation / Extracellular matrix / Glycoprotein / Metal-binding / Metalloprotease / Polymorphism / Protease / Secreted / Zinc / Zymogen | ||||||
| Function / homology |  Function and homology information Function and homology informationneutrophil collagenase / tumor necrosis factor binding / positive regulation of microglial cell activation / positive regulation of neuroinflammatory response / positive regulation of tumor necrosis factor-mediated signaling pathway / Activation of Matrix Metalloproteinases / endodermal cell differentiation / Collagen degradation / collagen catabolic process / extracellular matrix disassembly ...neutrophil collagenase / tumor necrosis factor binding / positive regulation of microglial cell activation / positive regulation of neuroinflammatory response / positive regulation of tumor necrosis factor-mediated signaling pathway / Activation of Matrix Metalloproteinases / endodermal cell differentiation / Collagen degradation / collagen catabolic process / extracellular matrix disassembly / Degradation of the extracellular matrix / extracellular matrix organization / metalloendopeptidase activity / specific granule lumen / positive regulation of tumor necrosis factor production / tertiary granule lumen / peptidase activity / : / cellular response to lipopolysaccharide / endopeptidase activity / serine-type endopeptidase activity / Neutrophil degranulation / proteolysis / extracellular space / extracellular region / zinc ion binding Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Pochetti, G. / Montanari, R. / Mazza, F. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2009 Journal: J.Med.Chem. / Year: 2009Title: Extra Binding Region Induced by Non-Zinc Chelating Inhibitors into the S(1)' Subsite of Matrix Metalloproteinase 8 (MMP-8) Authors: Pochetti, G. / Montanari, R. / Gege, C. / Chevrier, C. / Taveras, A.G. / Mazza, F. #1:  Journal: Chem.Biol. / Year: 2005 Journal: Chem.Biol. / Year: 2005Title: Structural basis for the highly selective inhibition of MMP-13 Authors: Engel, C.K. / Pirard, B. / Schimanski, S. / Kirsch, R. / Habermann, J. / Klingler, O. / Schlotte, V. / Weithmann, K.U. / Wendt, K.U. #2:  Journal: J.Biol.Chem. / Year: 2007 Journal: J.Biol.Chem. / Year: 2007Title: Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects Authors: Johnson, A.R. / Pavlovsky, A.G. / Ortwine, D.F. / Prior, F. / Man, C.F. / Bornemeier, D.A. / Banotai, C.A. / Mueller, W.T. / McConnell, P. / Yan, C. / Baragi, V. / Lesch, C. / Roark, W.H. / ...Authors: Johnson, A.R. / Pavlovsky, A.G. / Ortwine, D.F. / Prior, F. / Man, C.F. / Bornemeier, D.A. / Banotai, C.A. / Mueller, W.T. / McConnell, P. / Yan, C. / Baragi, V. / Lesch, C. / Roark, W.H. / Wilson, M. / Datta, K. / Guzman, R. / Han, H.K. / Dyer, R.D. #3:  Journal: J.Mol.Biol. / Year: 2004 Journal: J.Mol.Biol. / Year: 2004Title: Crystal structures of novel non-peptidic, non-zinc chelating inhibitors bound to MMP-12 Authors: Morales, R. / Perrier, S. / Florent, J.M. / Beltra, J. / Dufour, S. / De Mendez, I. / Manceau, P. / Tertre, A. / Moreau, F. / Compere, D. / Dublanchet, A.C. / O'Gara, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3dpf.cif.gz 3dpf.cif.gz | 91.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3dpf.ent.gz pdb3dpf.ent.gz | 68.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3dpf.json.gz 3dpf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  3dpf_validation.pdf.gz 3dpf_validation.pdf.gz | 902.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  3dpf_full_validation.pdf.gz 3dpf_full_validation.pdf.gz | 915.7 KB | Display | |
| Data in XML |  3dpf_validation.xml.gz 3dpf_validation.xml.gz | 22.1 KB | Display | |
| Data in CIF |  3dpf_validation.cif.gz 3dpf_validation.cif.gz | 31.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dp/3dpf https://data.pdbj.org/pub/pdb/validation_reports/dp/3dpf ftp://data.pdbj.org/pub/pdb/validation_reports/dp/3dpf ftp://data.pdbj.org/pub/pdb/validation_reports/dp/3dpf | HTTPS FTP |
-Related structure data
| Related structure data |  3dngC  3dpeC  1i76S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 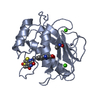
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 18111.744 Da / Num. of mol.: 2 / Fragment: MMP-8 catalytic domain, UNP residues 100-262 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MMP8, CLG1 / Plasmid: pSVB30 / Production host: Homo sapiens (human) / Gene: MMP8, CLG1 / Plasmid: pSVB30 / Production host:  |
|---|
-Non-polymers , 5 types, 437 molecules 







| #2: Chemical | ChemComp-CA / #3: Chemical | ChemComp-ZN / #4: Chemical | #5: Chemical | ChemComp-HAE / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.65 Å3/Da / Density % sol: 53.59 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 6 Details: Hanging droplets were made by mixing 1.5-3.0ml of protein/inhibitor solution with 5ml of PEG solution (10% PEG6000, 0.2M Mes-NaOH, 0.02% NaN3, pH6.0). Droplets were concentrated against a ...Details: Hanging droplets were made by mixing 1.5-3.0ml of protein/inhibitor solution with 5ml of PEG solution (10% PEG6000, 0.2M Mes-NaOH, 0.02% NaN3, pH6.0). Droplets were concentrated against a reservoir buffer containing 1.0-2.0M sodium phosphate, 0.02% NaN3, pH6.0, VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-2 / Wavelength: 0.873 Å / Beamline: ID23-2 / Wavelength: 0.873 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Jun 21, 2007 |
| Radiation | Monochromator: Si 111 channel / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.873 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→25 Å / Num. all: 22392 / Num. obs: 22392 / % possible obs: 96.9 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 4.1 % / Biso Wilson estimate: 18.7 Å2 / Rmerge(I) obs: 0.119 / Net I/σ(I): 4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1I76 Resolution: 2.1→10 Å / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→10 Å
| ||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj









