[English] 日本語
 Yorodumi
Yorodumi- PDB-3abw: Crystal structure of pharaonis halorhodopsin in complex with azide ion -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3abw | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of pharaonis halorhodopsin in complex with azide ion | ||||||
 Components Components | Halorhodopsin | ||||||
 Keywords Keywords | MEMBRANE PROTEIN / LIGHT-DRIVEN CHLORIDE ION PUMP / TRIMERIC BACTERIORUBERIN-PROTEIN COMPLEX / RETINAL PROTEIN | ||||||
| Function / homology | Rhopdopsin 7-helix transmembrane proteins / Rhodopsin 7-helix transmembrane proteins / Up-down Bundle / Mainly Alpha / BACTERIORUBERIN / AZIDE ION / Chem-L3P / RETINAL / :  Function and homology information Function and homology information | ||||||
| Biological species |  Natronomonas pharaonis (archaea) Natronomonas pharaonis (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||
 Authors Authors | Kouyama, T. / Kanada, S. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Binding mode of azide ion to pharaonis halorhodopsin Authors: Kanada, S. / Kouyama, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3abw.cif.gz 3abw.cif.gz | 169.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3abw.ent.gz pdb3abw.ent.gz | 131.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3abw.json.gz 3abw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ab/3abw https://data.pdbj.org/pub/pdb/validation_reports/ab/3abw ftp://data.pdbj.org/pub/pdb/validation_reports/ab/3abw ftp://data.pdbj.org/pub/pdb/validation_reports/ab/3abw | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3a7kS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 3 molecules ABD
| #1: Protein | Mass: 30975.096 Da / Num. of mol.: 3 / Source method: isolated from a natural source Details: STRAIN MK-1 WAS A HALORHODOPSIN-OVERPRODUCING MUTANT GENERATED FROM TYPE STRAIN D2160T. Source: (natural)  Natronomonas pharaonis (archaea) / Strain: MK-1 / References: UniProt: Q3ITX1 Natronomonas pharaonis (archaea) / Strain: MK-1 / References: UniProt: Q3ITX1 |
|---|
-Non-polymers , 5 types, 145 molecules 
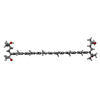
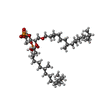






| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-L3P / #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.2 Å3/Da / Density % sol: 61.52 % |
|---|---|
| Crystal grow | Temperature: 288 K / Method: vapor diffusion, sitting drop / pH: 6 Details: A DROP CONTAINING 2MG/ML Halorhodopsin, 1M AMMONIUM SULFATE, 0.16M NACL, 0.04M Na-citrate, 0.04% NAN3,5MG/ML NONYL GLUCOSIDE WAS CONCENTRATED BY VAPOR DIFFUSIONAGAINST A RESERVE SOLUTION ...Details: A DROP CONTAINING 2MG/ML Halorhodopsin, 1M AMMONIUM SULFATE, 0.16M NACL, 0.04M Na-citrate, 0.04% NAN3,5MG/ML NONYL GLUCOSIDE WAS CONCENTRATED BY VAPOR DIFFUSIONAGAINST A RESERVE SOLUTION CONTAINING 2.9 M AMMONIUM SULFATE, 0.04M Na-citrate, PH 6, VAPOR DIFFUSION, SITTING DROP, TEMPERATURE 288K. After the crystal grew, the mother solution was exchanged with a solution containing 3M Ammonium sulfate, 200 mM Na-N3, 100 mM HEPES (pH7) and 30 % trehalose. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL38B1 / Wavelength: 1 Å / Beamline: BL38B1 / Wavelength: 1 Å |
| Detector | Type: RIGAKU JUPITER 210 / Detector: CCD / Date: Dec 14, 2009 / Details: mirrors |
| Radiation | Monochromator: Si 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→38.15 Å / Num. all: 91795 / Num. obs: 80799 / % possible obs: 88 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.2 % / Biso Wilson estimate: 23.3 Å2 / Rmerge(I) obs: 0.071 / Rsym value: 0.071 / Net I/σ(I): 10.5 |
| Reflection shell | Resolution: 1.9→2 Å / Redundancy: 3 % / Rmerge(I) obs: 0.526 / Mean I/σ(I) obs: 2.2 / Rsym value: 0.526 / % possible all: 74.3 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3a7k Resolution: 1.9→15 Å / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||||||||||
| Solvent computation | Bsol: 99.0968 Å2 | |||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 32.4952 Å2
| |||||||||||||||||||||||||||||||||
| Refine analyze |
| |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→15 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj









