[English] 日本語
 Yorodumi
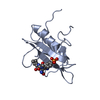
Yorodumi- PDB-2x18: The crystal structure of the PH domain of human AKT3 protein kinase -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2x18 | ||||||
|---|---|---|---|---|---|---|---|
| Title | The crystal structure of the PH domain of human AKT3 protein kinase | ||||||
 Components Components | RAC-GAMMA SERINE/THREONINE-PROTEIN KINASE | ||||||
 Keywords Keywords | TRANSFERASE / KINASE / MEMBRANE / ATP-BINDING | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of artery morphogenesis / AKT-mediated inactivation of FOXO1A / Negative regulation of the PI3K/AKT network / AKT phosphorylates targets in the nucleus / RUNX2 regulates genes involved in cell migration / : / RAB GEFs exchange GTP for GDP on RABs / brain morphogenesis / AKT phosphorylates targets in the cytosol / positive regulation of vascular endothelial cell proliferation ...positive regulation of artery morphogenesis / AKT-mediated inactivation of FOXO1A / Negative regulation of the PI3K/AKT network / AKT phosphorylates targets in the nucleus / RUNX2 regulates genes involved in cell migration / : / RAB GEFs exchange GTP for GDP on RABs / brain morphogenesis / AKT phosphorylates targets in the cytosol / positive regulation of vascular endothelial cell proliferation / positive regulation of cell migration involved in sprouting angiogenesis / Regulation of TP53 Activity through Association with Co-factors / Co-inhibition by CTLA4 / Constitutive Signaling by AKT1 E17K in Cancer / negative regulation of PERK-mediated unfolded protein response / negative regulation of cellular senescence / Regulation of localization of FOXO transcription factors / CD28 dependent PI3K/Akt signaling / positive regulation of blood vessel endothelial cell migration / positive regulation of TOR signaling / Estrogen-dependent nuclear events downstream of ESR-membrane signaling / positive regulation of cell size / Activation of BAD and translocation to mitochondria / SARS-CoV-2 targets host intracellular signalling and regulatory pathways / Cyclin E associated events during G1/S transition / Cyclin A:Cdk2-associated events at S phase entry / Downregulation of ERBB2:ERBB3 signaling / Regulation of TP53 Activity through Acetylation / positive regulation of endothelial cell proliferation / FLT3 Signaling / Transcriptional and post-translational regulation of MITF-M expression and activity / homeostasis of number of cells within a tissue / histone H4S1 kinase activity / : / histone H3S57 kinase activity / histone H2BS14 kinase activity / histone H3S28 kinase activity / histone H2AS1 kinase activity / histone H3T6 kinase activity / ribosomal protein S6 kinase activity / histone H2AT120 kinase activity / histone H2BS36 kinase activity / eukaryotic translation initiation factor 2alpha kinase activity / Rho-dependent protein serine/threonine kinase activity / histone H3S10 kinase activity / AMP-activated protein kinase activity / histone H3T11 kinase activity / histone H3T3 kinase activity / 3-phosphoinositide-dependent protein kinase activity / VEGFR2 mediated vascular permeability / histone H3T45 kinase activity / DNA-dependent protein kinase activity / histone H2AXS139 kinase activity / TP53 Regulates Metabolic Genes / Regulation of PTEN stability and activity / KEAP1-NFE2L2 pathway / positive regulation of angiogenesis / Regulation of TP53 Degradation / G beta:gamma signalling through PI3Kgamma / PIP3 activates AKT signaling / non-specific serine/threonine protein kinase / protein kinase activity / intracellular signal transduction / protein phosphorylation / ciliary basal body / cilium / protein serine kinase activity / protein serine/threonine kinase activity / Golgi apparatus / signal transduction / nucleoplasm / ATP binding / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.46 Å MOLECULAR REPLACEMENT / Resolution: 1.46 Å | ||||||
 Authors Authors | Vollmar, M. / Wang, J. / Zhang, Y. / Elkins, J.M. / Burgess-Brown, N. / Chaikuad, A. / Pike, A.C.W. / von Delft, F. / Bountra, C. / Arrowsmith, C.H. ...Vollmar, M. / Wang, J. / Zhang, Y. / Elkins, J.M. / Burgess-Brown, N. / Chaikuad, A. / Pike, A.C.W. / von Delft, F. / Bountra, C. / Arrowsmith, C.H. / Weigelt, J. / Edwards, A. / Knapp, S. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: The Crystal Structure of the Ph Domain of Human Akt3 Protein Kinase Authors: Vollmar, M. / Wang, J. / Zhang, Y. / Elkins, J.M. / Burgess-Brown, N. / Chaikuad, A. / Pike, A.C.W. / von Delft, F. / Bountra, C. / Arrowsmith, C.H. / Weigelt, J. / Edwards, A. / Knapp, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2x18.cif.gz 2x18.cif.gz | 239.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2x18.ent.gz pdb2x18.ent.gz | 194.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2x18.json.gz 2x18.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2x18_validation.pdf.gz 2x18_validation.pdf.gz | 503 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2x18_full_validation.pdf.gz 2x18_full_validation.pdf.gz | 517.1 KB | Display | |
| Data in XML |  2x18_validation.xml.gz 2x18_validation.xml.gz | 57.1 KB | Display | |
| Data in CIF |  2x18_validation.cif.gz 2x18_validation.cif.gz | 85.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/x1/2x18 https://data.pdbj.org/pub/pdb/validation_reports/x1/2x18 ftp://data.pdbj.org/pub/pdb/validation_reports/x1/2x18 ftp://data.pdbj.org/pub/pdb/validation_reports/x1/2x18 | HTTPS FTP |
-Related structure data
| Related structure data |  1unpS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 | 
| ||||||||
| 7 | 
| ||||||||
| 8 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 14288.233 Da / Num. of mol.: 8 / Fragment: PH DOMAIN, RESIDUES 466-583 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PNIC28-BSA4 / Production host: HOMO SAPIENS (human) / Plasmid: PNIC28-BSA4 / Production host:  References: UniProt: Q9Y243, non-specific serine/threonine protein kinase #2: Chemical | ChemComp-EPE / #3: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.45 Å3/Da / Density % sol: 49.81 % / Description: NONE |
|---|---|
| Crystal grow | Details: 0.2M PROLINE, 0.1M HEPES PH 7.5, 10% PEG3350 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.976 / Beamline: I04 / Wavelength: 0.976 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Oct 21, 2009 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.976 Å / Relative weight: 1 |
| Reflection | Resolution: 1.46→64.03 Å / Num. obs: 164858 / % possible obs: 98.1 % / Observed criterion σ(I): 0 / Redundancy: 3.5 % / Biso Wilson estimate: 15.1 Å2 / Rmerge(I) obs: 0.08 / Net I/σ(I): 10 |
| Reflection shell | Resolution: 1.46→1.54 Å / Redundancy: 2.7 % / Rmerge(I) obs: 0.47 / Mean I/σ(I) obs: 2 / % possible all: 94.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1UNP Resolution: 1.46→64.03 Å / Cor.coef. Fo:Fc: 0.961 / Cor.coef. Fo:Fc free: 0.94 / SU B: 3.003 / SU ML: 0.052 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / ESU R: 0.08 / ESU R Free: 0.086 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 10.825 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.46→64.03 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj























