[English] 日本語
 Yorodumi
Yorodumi- PDB-2pf8: Complex of Aldose Reductase with NADP+ and simaltaneously bound c... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2pf8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Complex of Aldose Reductase with NADP+ and simaltaneously bound competetive inhibitors Fidarestat and IDD594. Concentration of Fidarestat in soaking solution is equal to concentration of IDD594. | ||||||
 Components Components | Aldose reductase | ||||||
 Keywords Keywords | OXIDOREDUCTASE / NADP / IDD594 / FIDARESTAT | ||||||
| Function / homology |  Function and homology information Function and homology informationglyceraldehyde oxidoreductase activity / Fructose biosynthesis / fructose biosynthetic process / L-glucuronate reductase activity / aldose reductase / D/L-glyceraldehyde reductase / glycerol dehydrogenase (NADP+) activity / C21-steroid hormone biosynthetic process / NADP-retinol dehydrogenase / Pregnenolone biosynthesis ...glyceraldehyde oxidoreductase activity / Fructose biosynthesis / fructose biosynthetic process / L-glucuronate reductase activity / aldose reductase / D/L-glyceraldehyde reductase / glycerol dehydrogenase (NADP+) activity / C21-steroid hormone biosynthetic process / NADP-retinol dehydrogenase / Pregnenolone biosynthesis / allyl-alcohol dehydrogenase / allyl-alcohol dehydrogenase activity / prostaglandin H2 endoperoxidase reductase activity / regulation of urine volume / metanephric collecting duct development / all-trans-retinol dehydrogenase (NADP+) activity / daunorubicin metabolic process / doxorubicin metabolic process / retinal dehydrogenase (NAD+) activity / aldose reductase (NADPH) activity / epithelial cell maturation / cellular hyperosmotic salinity response / retinoid metabolic process / renal water homeostasis / carbohydrate metabolic process / electron transfer activity / negative regulation of apoptotic process / mitochondrion / extracellular space / extracellular exosome / nucleoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 0.85 Å MOLECULAR REPLACEMENT / Resolution: 0.85 Å | ||||||
 Authors Authors | Petrova, T. / Hazemann, I. / Cousido, A. / Mitschler, A. / Ginell, S. / Joachimiak, A. / Podjarny, A. | ||||||
 Citation Citation |  Journal: Proteins / Year: 2012 Journal: Proteins / Year: 2012Title: Crystal packing modifies ligand binding affinity: The case of aldose reductase. Authors: Cousido-Siah, A. / Petrova, T. / Hazemann, I. / Mitschler, A. / Ruiz, F.X. / Howard, E. / Ginell, S. / Atmanene, C. / Van Dorsselaer, A. / Sanglier-Cianferani, S. / Joachimiak, A. / Podjarny, A. | ||||||
| History |
| ||||||
| Remark 999 | SEQUENCE THE CONFLICT ANNOTATED IN THE SEQADV CARDS BELOW HAS BEEN DESCRIBED IN J.BIOL.CHEM. 264: ...SEQUENCE THE CONFLICT ANNOTATED IN THE SEQADV CARDS BELOW HAS BEEN DESCRIBED IN J.BIOL.CHEM. 264:14775 (1989). |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2pf8.cif.gz 2pf8.cif.gz | 208 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2pf8.ent.gz pdb2pf8.ent.gz | 164.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2pf8.json.gz 2pf8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2pf8_validation.pdf.gz 2pf8_validation.pdf.gz | 1.3 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2pf8_full_validation.pdf.gz 2pf8_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  2pf8_validation.xml.gz 2pf8_validation.xml.gz | 25.2 KB | Display | |
| Data in CIF |  2pf8_validation.cif.gz 2pf8_validation.cif.gz | 40.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pf/2pf8 https://data.pdbj.org/pub/pdb/validation_reports/pf/2pf8 ftp://data.pdbj.org/pub/pdb/validation_reports/pf/2pf8 ftp://data.pdbj.org/pub/pdb/validation_reports/pf/2pf8 | HTTPS FTP |
-Related structure data
| Related structure data |  2pevC  2pfhC  1us0S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 35898.340 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: AKR1B1, ALDR1 / Production host: Homo sapiens (human) / Gene: AKR1B1, ALDR1 / Production host:  |
|---|
-Non-polymers , 6 types, 730 molecules 

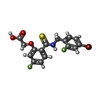








| #2: Chemical | ChemComp-CL / | ||
|---|---|---|---|
| #3: Chemical | ChemComp-NDP / | ||
| #4: Chemical | ChemComp-LDT / | ||
| #5: Chemical | ChemComp-FID / ( | ||
| #6: Chemical | | #7: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.17 Å3/Da / Density % sol: 43.39 % |
|---|---|
| Crystal grow | Temperature: 297 K / Method: vapor diffusion, hanging drop / pH: 5 Details: micro-seeding, pH 5, VAPOR DIFFUSION, HANGING DROP, temperature 297K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.65255 Å / Beamline: 19-ID / Wavelength: 0.65255 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jan 1, 2000 |
| Radiation | Monochromator: DOUBLE CRYSTAL MONOCHROMATOR UTILIZING A SI-111 AND SAGITAL HORIZONTAL FOCUSING Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.65255 Å / Relative weight: 1 |
| Reflection | Resolution: 0.85→50 Å / Num. all: 267837 / Num. obs: 254426 / % possible obs: 99.7 % / Rmerge(I) obs: 0.085 / Net I/σ(I): 16.41 |
| Reflection shell | Resolution: 0.85→0.88 Å / Rmerge(I) obs: 0.168 / Mean I/σ(I) obs: 4.52 / % possible all: 98.72 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1US0 Resolution: 0.85→50 Å / Num. parameters: 36921 / Num. restraintsaints: 55747 / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: ENGH AND HUBER
| |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 499 / Occupancy sum hydrogen: 2288.45 / Occupancy sum non hydrogen: 3038.8 | |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 0.85→50 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


















 PDBj
PDBj







