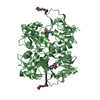
Entry Database : PDB / ID : 2nz8Title N-terminal DHPH cassette of Trio in complex with nucleotide-free Rac1 ras-related C3 botulinum toxin substrate 1 isoform Rac1 triple functional domain protein Keywords / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 2 Å Authors Chhatriwala, M.K. / Betts, L. / Worthylake, D.K. / Sondek, J. Journal : J.Mol.Biol. / Year : 2007Title : The DH and PH Domains of Trio Coordinately Engage Rho GTPases for their Efficient ActivationAuthors : Chhatriwala, M.K. / Betts, L. / Worthylake, D.K. / Sondek, J. History Deposition Nov 22, 2006 Deposition site / Processing site Revision 1.0 Apr 10, 2007 Provider / Type Revision 1.1 May 1, 2008 Group Revision 1.2 Jul 13, 2011 Group Revision 1.3 Oct 18, 2017 Group / Category Revision 1.4 Aug 30, 2023 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / struct_ref_seq_dif Item / _database_2.pdbx_database_accession / _struct_ref_seq_dif.details
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å
MOLECULAR REPLACEMENT / Resolution: 2 Å  Authors
Authors Citation
Citation Journal: J.Mol.Biol. / Year: 2007
Journal: J.Mol.Biol. / Year: 2007 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2nz8.cif.gz
2nz8.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2nz8.ent.gz
pdb2nz8.ent.gz PDB format
PDB format 2nz8.json.gz
2nz8.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/nz/2nz8
https://data.pdbj.org/pub/pdb/validation_reports/nz/2nz8 ftp://data.pdbj.org/pub/pdb/validation_reports/nz/2nz8
ftp://data.pdbj.org/pub/pdb/validation_reports/nz/2nz8 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: RAC1 / Plasmid: pET15b / Species (production host): Escherichia coli / Production host:
Homo sapiens (human) / Gene: RAC1 / Plasmid: pET15b / Species (production host): Escherichia coli / Production host: 
 Homo sapiens (human) / Gene: TRIO / Plasmid: pPROEX-HTa / Species (production host): Escherichia coli / Production host:
Homo sapiens (human) / Gene: TRIO / Plasmid: pPROEX-HTa / Species (production host): Escherichia coli / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 22-ID / Wavelength: 1.0712 Å
/ Beamline: 22-ID / Wavelength: 1.0712 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller













 PDBj
PDBj



















