[English] 日本語
 Yorodumi
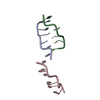
Yorodumi- PDB-2ic6: The Coiled-coil Domain (residues 1-75) Structure of the Sin Nombr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2ic6 | ||||||
|---|---|---|---|---|---|---|---|
| Title | The Coiled-coil Domain (residues 1-75) Structure of the Sin Nombre Virus Nucleocapsid Protein | ||||||
 Components Components | Nucleocapsid protein | ||||||
 Keywords Keywords | VIRAL PROTEIN / Sin Nombre Virus / Hantavirus / Bunyaviridae / ssRNA negative-strand viruses / nucleocapsid protein / antiparallel coiled coil | ||||||
| Function / homology |  Function and homology information Function and homology informationviral nucleocapsid / endonuclease activity / host cell Golgi apparatus / Hydrolases; Acting on ester bonds / host cell perinuclear region of cytoplasm / ribonucleoprotein complex / hydrolase activity / RNA binding Similarity search - Function | ||||||
| Biological species |  Sin Nombre virus Sin Nombre virus | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 1.15 Å MAD / Resolution: 1.15 Å | ||||||
 Authors Authors | Boudko, S.P. / Rossmann, M.G. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2007 Journal: J.Mol.Biol. / Year: 2007Title: The Coiled-coil Domain Structure of the Sin Nombre Virus Nucleocapsid Protein. Authors: Boudko, S.P. / Kuhn, R.J. / Rossmann, M.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2ic6.cif.gz 2ic6.cif.gz | 80.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2ic6.ent.gz pdb2ic6.ent.gz | 61.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2ic6.json.gz 2ic6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ic/2ic6 https://data.pdbj.org/pub/pdb/validation_reports/ic/2ic6 ftp://data.pdbj.org/pub/pdb/validation_reports/ic/2ic6 ftp://data.pdbj.org/pub/pdb/validation_reports/ic/2ic6 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 8708.889 Da / Num. of mol.: 2 / Fragment: Residues: 1-75 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Sin Nombre virus / Genus: Hantavirus / Strain: Convict Creek 107 virus / Gene: nucleocapsid protein / Plasmid: pET23d(+) / Species (production host): Escherichia coli / Production host: Sin Nombre virus / Genus: Hantavirus / Strain: Convict Creek 107 virus / Gene: nucleocapsid protein / Plasmid: pET23d(+) / Species (production host): Escherichia coli / Production host:  #2: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.01 Å3/Da / Density % sol: 38.78 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 4.6 Details: 0.2 M ammonium acetate, 0.1 M sodium acetate, 30% polyethylene glycol (PEG) 4000, pH 4.6, VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | D res high: 1.33 Å / D res low: 50 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diffraction reflection shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.15→10 Å / Num. all: 50803 / Num. obs: 50773 / % possible obs: 99.9 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 2 / Redundancy: 5.8 % / Biso Wilson estimate: 9 Å2 / Rmerge(I) obs: 0.051 / Rsym value: 0.051 / Χ2: 1.03 / Net I/σ(I): 27.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Resolution: 1.15→1.17 Å / Redundancy: 4.3 % / Rmerge(I) obs: 0.238 / Mean I/σ(I) obs: 6.6 / Num. unique all: 2516 / Rsym value: 0.238 / Χ2: 1.039 / % possible all: 99.8 |
-Phasing
| Phasing | Method:  MAD MAD | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phasing set |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing MAD set |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing MAD set site | Atom type symbol: Se / B iso: 12.405 / Fract x: 0.171 / Fract y: 0.09 / Fract z: 0.058 / Occupancy: 0.692 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing dm | FOM : 0.78 / FOM acentric: 0.78 / FOM centric: 0.76 / Reflection: 29724 / Reflection acentric: 26983 / Reflection centric: 2741 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing dm shell |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MAD / Resolution: 1.15→10 Å / Cor.coef. Fo:Fc: 0.971 / Cor.coef. Fo:Fc free: 0.967 / SU B: 0.982 / SU ML: 0.021 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.036 / ESU R Free: 0.036 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS MAD / Resolution: 1.15→10 Å / Cor.coef. Fo:Fc: 0.971 / Cor.coef. Fo:Fc free: 0.967 / SU B: 0.982 / SU ML: 0.021 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.036 / ESU R Free: 0.036 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 10.85 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.15→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.15→1.18 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller













 PDBj
PDBj

