[English] 日本語
 Yorodumi
Yorodumi- PDB-1sln: CRYSTAL STRUCTURE OF THE CATALYTIC DOMAIN OF HUMAN FIBROBLAST STR... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1sln | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
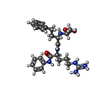
| Title | CRYSTAL STRUCTURE OF THE CATALYTIC DOMAIN OF HUMAN FIBROBLAST STROMELYSIN-1 INHIBITED WITH THE N-CARBOXY-ALKYL INHIBITOR L-702,842 | |||||||||
 Components Components | STROMELYSIN-1 | |||||||||
 Keywords Keywords | HYDROLASE / METALLOPROTEASE / FIBROBLAST / COLLAGEN DEGRADATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationstromelysin 1 / cellular response to UV-A / regulation of neuroinflammatory response / Assembly of collagen fibrils and other multimeric structures / Activation of Matrix Metalloproteinases / Collagen degradation / response to amyloid-beta / collagen catabolic process / negative regulation of reactive oxygen species metabolic process / extracellular matrix disassembly ...stromelysin 1 / cellular response to UV-A / regulation of neuroinflammatory response / Assembly of collagen fibrils and other multimeric structures / Activation of Matrix Metalloproteinases / Collagen degradation / response to amyloid-beta / collagen catabolic process / negative regulation of reactive oxygen species metabolic process / extracellular matrix disassembly / Degradation of the extracellular matrix / extracellular matrix organization / EGFR Transactivation by Gastrin / cellular response to nitric oxide / regulation of cell migration / negative regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / cellular response to reactive oxygen species / protein catabolic process / cellular response to amino acid stimulus / positive regulation of protein-containing complex assembly / metalloendopeptidase activity / extracellular matrix / metallopeptidase activity / peptidase activity / cellular response to lipopolysaccharide / Interleukin-4 and Interleukin-13 signaling / endopeptidase activity / Extra-nuclear estrogen signaling / serine-type endopeptidase activity / innate immune response / mitochondrion / proteolysis / extracellular space / extracellular region / zinc ion binding / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.27 Å X-RAY DIFFRACTION / Resolution: 2.27 Å | |||||||||
 Authors Authors | Becker, J.W. | |||||||||
 Citation Citation |  Journal: Protein Sci. / Year: 1995 Journal: Protein Sci. / Year: 1995Title: Stromelysin-1: three-dimensional structure of the inhibited catalytic domain and of the C-truncated proenzyme. Authors: Becker, J.W. / Marcy, A.I. / Rokosz, L.L. / Axel, M.G. / Burbaum, J.J. / Fitzgerald, P.M. / Cameron, P.M. / Esser, C.K. / Hagmann, W.K. / Hermes, J.D. / Springer, J.P. #1:  Journal: Nat.Struct.Biol. / Year: 1994 Journal: Nat.Struct.Biol. / Year: 1994Title: The NMR Structure of the Inhibited Catalytic Domain of Human Stromelysin-1 Authors: Gooley, P.R. / O'Connell, J.F. / Marcy, A.I. / Cuca, G.C. / Salowe, S.P. / Bush, B.L. / Hermes, J.D. / Esser, C.K. / Hagmann, W.K. / Springer, J.P. / Johnson, B.A. #2:  Journal: J.Med.Chem. / Year: 1993 Journal: J.Med.Chem. / Year: 1993Title: Inhibition of Matrix Metalloproteinases by N-Carboxyalkyl Peptides Authors: Chapman, K.T. / Kopka, I.E. / Durette, P.L. / Esser, C.K. / Lanza, T.J. / Izquierdo-Martin, M. / Niedzwiecki, L. / Chang, B. / Harrison, R.K. / Kuo, D.W. / Lin, T.-T. / Stein, R.L. / Hagmann, W.K. #3:  Journal: Biochemistry / Year: 1991 Journal: Biochemistry / Year: 1991Title: Human Fibroblast Stromelysin Catalytic Domain: Expression, Purification, and Characterization of a C-Terminally Truncated Form Authors: Marcy, A.I. / Eiberger, L.L. / Harrison, R. / Chan, H.K. / Hutchinson, N.I. / Hagmann, W.K. / Cameron, P.M. / Boulton, D.A. / Hermes, J.D. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1sln.cif.gz 1sln.cif.gz | 59.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1sln.ent.gz pdb1sln.ent.gz | 41.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1sln.json.gz 1sln.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sl/1sln https://data.pdbj.org/pub/pdb/validation_reports/sl/1sln ftp://data.pdbj.org/pub/pdb/validation_reports/sl/1sln ftp://data.pdbj.org/pub/pdb/validation_reports/sl/1sln | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 19416.529 Da / Num. of mol.: 1 / Fragment: CATALYTIC DOMAIN RESIDUES 83 - 255 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell: FIBROBLAST / Production host: Homo sapiens (human) / Cell: FIBROBLAST / Production host:  | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-8MI / | #5: Water | ChemComp-HOH / | Compound details | THERE IS A HYDROGEN BOND BETWEEN THE NITROGEN ATOM OF ALA 140 AND THE S-DELTA OF MET 143. | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.45 Å3/Da / Density % sol: 49.3 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, hanging drop / pH: 5.54 Details: HANGING DROP VAPOR DIFFUSION. 1.5 MICROLITER DROPS OF ENZYME:INHIBITOR SOLUTION (9 MG/ML ENZYME, 1.5 MILLIMOLAR INHIBITOR, 5.0 MILLIMOLAR CALCIUM CHLORIDE, 0.02% SODIUM AZIDE, 20 MILLIMOLAR ...Details: HANGING DROP VAPOR DIFFUSION. 1.5 MICROLITER DROPS OF ENZYME:INHIBITOR SOLUTION (9 MG/ML ENZYME, 1.5 MILLIMOLAR INHIBITOR, 5.0 MILLIMOLAR CALCIUM CHLORIDE, 0.02% SODIUM AZIDE, 20 MILLIMOLAR TRIS HYDROCHLORIDE, PH 7.5) WERE MIXED WITH AN EQUAL VOLUME OF RESERVOIR BUFFER (10% PEG-6000, 15% SATURATED AMMONIUM ACETATE 0.02% SODIUM AZIDE, 0.1 M CACODYLATE, PH 5.54) AND INCUBATED AT ROOM TEMPERATURE., vapor diffusion - hanging drop PH range: 5.54-7.5 / Temp details: room temp | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Source: SEALED TUBE / Wavelength: 1.5418 |
| Detector | Type: SIEMENS / Detector: AREA DETECTOR / Date: Dec 1, 1990 |
| Radiation | Monochromator: GRAPHITE(002) / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.27→8 Å / Num. obs: 8311 / % possible obs: 88.9 % / Observed criterion σ(I): 1 / Redundancy: 2.79 % / Biso Wilson estimate: 11.76 Å2 / Rmerge(I) obs: 0.0598 / Rsym value: 0.0758 / Net I/σ(I): 11.9 |
| Reflection shell | Resolution: 2.26→2.41 Å / Redundancy: 1.71 % / Mean I/σ(I) obs: 4.03 / Rsym value: 0.189 / % possible all: 60.9 |
| Reflection shell | *PLUS % possible obs: 60.9 % / Rmerge(I) obs: 0.189 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.27→8 Å / Cross valid method: FREE-R / σ(F): 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 11.69 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.27→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.27→2.37 Å / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj