[English] 日本語
 Yorodumi
Yorodumi- PDB-1omd: STRUCTURE OF ONCOMODULIN REFINED AT 1.85 ANGSTROMS RESOLUTION. AN... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1omd | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF ONCOMODULIN REFINED AT 1.85 ANGSTROMS RESOLUTION. AN EXAMPLE OF EXTENSIVE MOLECULAR AGGREGATION VIA CA2+ | ||||||
 Components Components | ONCOMODULIN | ||||||
 Keywords Keywords | CALCIUM BINDING PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationcuticular plate / stereocilium / supramolecular fiber / cochlea development / response to wounding / vesicle / calcium ion binding / protein-containing complex binding / protein homodimerization activity / protein-containing complex ...cuticular plate / stereocilium / supramolecular fiber / cochlea development / response to wounding / vesicle / calcium ion binding / protein-containing complex binding / protein homodimerization activity / protein-containing complex / extracellular space / identical protein binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.85 Å X-RAY DIFFRACTION / Resolution: 1.85 Å | ||||||
 Authors Authors | Ahmed, F.R. / Przybylska, M. / Rose, D.R. / Birnbaum, G.I. / Pippy, M.E. / Macmanus, J.P. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1990 Journal: J.Mol.Biol. / Year: 1990Title: Structure of oncomodulin refined at 1.85 A resolution. An example of extensive molecular aggregation via Ca2+. Authors: Ahmed, F.R. / Przybylska, M. / Rose, D.R. / Birnbaum, G.I. / Pippy, M.E. / MacManus, J.P. #1:  Journal: J.Mol.Biol. / Year: 1988 Journal: J.Mol.Biol. / Year: 1988Title: Crystallization and Preliminary Crystallographic Data for Oncomodulin Authors: Przybylska, M. / Ahmed, F.R. / Birnbaum, G.I. / Rose, D.R. #2:  Journal: J.Biol.Chem. / Year: 1987 Journal: J.Biol.Chem. / Year: 1987Title: A Complete Complementary DNA for the Oncodevelopmental Calcium-Binding Protein, Oncomodulin Authors: Gillen, M.F. / Banville, D. / Rutledge, R.G. / Narang, S. / Seligy, V.L. / Whitfield, J.F. / Macmanus, J.P. #3:  Journal: Eur.J.Biochem. / Year: 1983 Journal: Eur.J.Biochem. / Year: 1983Title: The Complete Amino Acid Sequence of Oncomodulin-A Parvalbumin-Like Calcium-Binding Protein from Morris Hepatoma 5123Tc Authors: Macmanus, J.P. / Watson, D.C. / Yaguchi, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
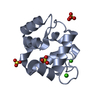
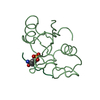
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1omd.cif.gz 1omd.cif.gz | 36.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1omd.ent.gz pdb1omd.ent.gz | 24.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1omd.json.gz 1omd.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/om/1omd https://data.pdbj.org/pub/pdb/validation_reports/om/1omd ftp://data.pdbj.org/pub/pdb/validation_reports/om/1omd ftp://data.pdbj.org/pub/pdb/validation_reports/om/1omd | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 12067.064 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  | ||||
|---|---|---|---|---|---|
| #2: Chemical | | #3: Water | ChemComp-HOH / | Nonpolymer details | CALCIUM 135 IS INTERMOLECULAR. IT IS BONDED DIRECTLY TO THREE DIFFERENT ONCOMODULIN MOLECULES AND ...CALCIUM 135 IS INTERMOLEC | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.74 Å3/Da / Density % sol: 29.45 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 5.2 / Method: vapor diffusion, hanging drop / Details: took Przybylska et al., from original paper | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 1.85 Å / Num. obs: 5965 / Rmerge(I) obs: 3 |
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.85→6 Å /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: PROLSQ / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.166 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 2 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller










 PDBj
PDBj


