[English] 日本語
 Yorodumi
Yorodumi- PDB-1oha: Acetylglutamate kinase from Escherichia coli complexed with MgADP... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1oha | ||||||
|---|---|---|---|---|---|---|---|
| Title | Acetylglutamate kinase from Escherichia coli complexed with MgADP and N-acetyl-L-glutamate | ||||||
 Components Components | ACETYLGLUTAMATE KINASE | ||||||
 Keywords Keywords | KINASE / N-ACETYL-L-GLUTAMATE KINASE / AMINO ACID KINASE / PHOSPHORYL N-ACETYL-L-GLUTAMATE 5-PHOSPHOTRANSFERASE / NAG KINASE / GROUP TRANSFER / ARGININE METABOLISM | ||||||
| Function / homology |  Function and homology information Function and homology informationacetylglutamate kinase / acetylglutamate kinase activity / L-arginine biosynthetic process via ornithine / L-arginine biosynthetic process / DNA damage response / ATP binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||
 Authors Authors | Gil-Ortiz, F. / Ramon-Maiques, S. / Fita, I. / Rubio, V. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2003 Journal: J.Mol.Biol. / Year: 2003Title: The Course of Phosphorus in the Reaction of N-Acetyl-L-Glutamate Kinase, Determined from the Structures of Crystalline Complexes, Including a Complex with an Alf(4)(-) Transition State Mimic Authors: Gil-Ortiz, F. / Ramon-Maiques, S. / Fita, I. / Rubio, V. | ||||||
| History |
| ||||||
| Remark 650 | HELIX DETERMINATION METHOD: AUTHOR PROVIDED. | ||||||
| Remark 700 | SHEET DETERMINATION METHOD: AUTHOR PROVIDED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1oha.cif.gz 1oha.cif.gz | 69.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1oha.ent.gz pdb1oha.ent.gz | 49.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1oha.json.gz 1oha.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oh/1oha https://data.pdbj.org/pub/pdb/validation_reports/oh/1oha ftp://data.pdbj.org/pub/pdb/validation_reports/oh/1oha ftp://data.pdbj.org/pub/pdb/validation_reports/oh/1oha | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1oh9C  1ohbC  1gs5S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 27186.492 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P11445, UniProt: P0A6C8*PLUS, acetylglutamate kinase |
|---|
-Non-polymers , 5 types, 145 molecules 
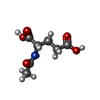







| #2: Chemical | ChemComp-ADP / |
|---|---|
| #3: Chemical | ChemComp-NLG / |
| #4: Chemical | ChemComp-ACT / |
| #5: Chemical | ChemComp-MG / |
| #6: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.1 Å3/Da / Density % sol: 41 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 4.6 Details: 27-29% POLYETHYLENE GLYCOL MONOMETHYL ETHER 2K, SODIUM ACETATE 0.1M PH 4.6 AMMONIUM SULFATE 0.15-0.3 M | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 4.6 / Method: vapor diffusion | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-2 / Wavelength: 0.934 / Beamline: ID14-2 / Wavelength: 0.934 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: May 10, 2000 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.934 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→19.43 Å / Num. obs: 18636 / % possible obs: 99.9 % / Observed criterion σ(I): 0 / Redundancy: 8.3 % / Biso Wilson estimate: 18.1 Å2 / Rmerge(I) obs: 0.068 / Net I/σ(I): 7.3 |
| Reflection shell | Resolution: 1.9→2 Å / Redundancy: 6 % / Rmerge(I) obs: 0.469 / Mean I/σ(I) obs: 1.3 / % possible all: 99.6 |
| Reflection | *PLUS Highest resolution: 1.9 Å / Lowest resolution: 19.43 Å / Num. measured all: 154986 / Rmerge(I) obs: 0.068 |
| Reflection shell | *PLUS % possible obs: 99.6 % / Rmerge(I) obs: 0.469 / Mean I/σ(I) obs: 1.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1GS5 Resolution: 1.9→19.43 Å / Rfactor Rfree error: 0.007 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 51.2273 Å2 / ksol: 0.375726 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 26.6 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→19.43 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.9→2.02 Å / Rfactor Rfree error: 0.021 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Num. reflection obs: 17671 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller













 PDBj
PDBj




