[English] 日本語
 Yorodumi
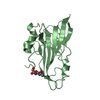
Yorodumi- PDB-1nu2: Crystal structure of the murine Disabled-1 (Dab1) PTB domain-ApoE... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1nu2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the murine Disabled-1 (Dab1) PTB domain-ApoER2 peptide-PI-4,5P2 ternary complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | SIGNALING PROTEIN / beta-sandwich | ||||||
| Function / homology |  Function and homology information Function and homology informationcell-cell adhesion involved in neuronal-glial interactions involved in cerebral cortex radial glia guided migration / lateral motor column neuron migration / Reelin signalling pathway / radial glia guided migration of Purkinje cell / Golgi localization / cerebellum structural organization / cerebral cortex radially oriented cell migration / negative regulation of axonogenesis / ventral spinal cord development / reelin-mediated signaling pathway ...cell-cell adhesion involved in neuronal-glial interactions involved in cerebral cortex radial glia guided migration / lateral motor column neuron migration / Reelin signalling pathway / radial glia guided migration of Purkinje cell / Golgi localization / cerebellum structural organization / cerebral cortex radially oriented cell migration / negative regulation of axonogenesis / ventral spinal cord development / reelin-mediated signaling pathway / negative regulation of receptor signaling pathway via JAK-STAT / regulation of synapse maturation / negative regulation of cell adhesion / adult walking behavior / small GTPase-mediated signal transduction / negative regulation of astrocyte differentiation / cerebral cortex cell migration / dendrite development / brush border / phosphatidylinositol 3-kinase binding / positive regulation of neuron differentiation / signaling adaptor activity / SH2 domain binding / central nervous system development / hippocampus development / phospholipid binding / neuron migration / apical part of cell / nervous system development / intracellular signal transduction / neuronal cell body / synapse / perinuclear region of cytoplasm / glutamatergic synapse / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||
 Authors Authors | Stolt, P.C. / Jeon, H. / Song, H.K. / Herz, J. / Eck, M.J. / Blacklow, S.C. | ||||||
 Citation Citation |  Journal: Structure / Year: 2003 Journal: Structure / Year: 2003Title: Origins of Peptide Selectivity and Phosphoinositide Binding Revealed by Structures of Disabled-1 PTB Domain Complexes Authors: Stolt, P.C. / Jeon, H. / Song, H.K. / Herz, J. / Eck, M.J. / Blacklow, S.C. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1nu2.cif.gz 1nu2.cif.gz | 49 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1nu2.ent.gz pdb1nu2.ent.gz | 34.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1nu2.json.gz 1nu2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nu/1nu2 https://data.pdbj.org/pub/pdb/validation_reports/nu/1nu2 ftp://data.pdbj.org/pub/pdb/validation_reports/nu/1nu2 ftp://data.pdbj.org/pub/pdb/validation_reports/nu/1nu2 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1ntvSC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 17084.768 Da / Num. of mol.: 1 / Fragment: residues 23-174 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Protein/peptide | Mass: 1255.379 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: created by peptide synthesis |
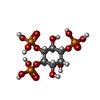
| #3: Chemical | ChemComp-I3P / |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.8 Å3/Da / Density % sol: 31.08 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / pH: 7.5 Details: HEPES, PEG8000, ethanol, pH 7.5, VAPOR DIFFUSION, HANGING DROP, temperature 298K, pH 7.50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 7.5 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Nov 29, 2002 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→40 Å / Num. obs: 12179 / % possible obs: 98 % / Observed criterion σ(I): -3 / Biso Wilson estimate: 17 Å2 / Rmerge(I) obs: 0.047 |
| Reflection shell | Resolution: 1.9→1.96 Å / Rmerge(I) obs: 0.17 / % possible all: 97.5 |
| Reflection | *PLUS Lowest resolution: 40 Å / % possible obs: 98 % / Num. measured all: 34852 |
| Reflection shell | *PLUS % possible obs: 97.5 % / Rmerge(I) obs: 0.17 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1NTV Resolution: 1.9→40 Å / Rfactor Rfree error: 0.007 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 53.9985 Å2 / ksol: 0.383678 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 25.6 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→40 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.9→1.96 Å / Rfactor Rfree error: 0.029 / Total num. of bins used: 12
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 1.9 Å / Lowest resolution: 40 Å / % reflection Rfree: 10 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj