[English] 日本語
 Yorodumi
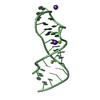
Yorodumi- PDB-1msy: GUAA tetraloop mutant of Sarcin/Ricin domain from E. Coli 23 S rRNA -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1msy | ||||||
|---|---|---|---|---|---|---|---|
| Title | GUAA tetraloop mutant of Sarcin/Ricin domain from E. Coli 23 S rRNA | ||||||
 Components Components | SARCIN/RICIN DOMAIN FROM 23 S RRNA | ||||||
 Keywords Keywords | RNA / A-minor motif / sarcin/ricin loop / RNA structure / RNA tertiary contacts / RNA motifs / conformational change / RNA-protein recognition / hydration | ||||||
| Function / homology | RNA / RNA (> 10) Function and homology information Function and homology information | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.41 Å MOLECULAR REPLACEMENT / Resolution: 1.41 Å | ||||||
 Authors Authors | Correll, C.C. / Swinger, K. | ||||||
 Citation Citation |  Journal: RNA / Year: 2003 Journal: RNA / Year: 2003Title: Common and distinctive features of GNRA tetraloops based on a GUAA tetraloop structure at 1.4 A resolution Authors: Correll, C.C. / Swinger, K. | ||||||
| History |
| ||||||
| Remark 400 | COMPOUND A CRYSTALLOGRAPHIC TWO-FOLD AXIS PASSES THROUGH THE TERMINAL GU WOBBLEBASE PAIR. EACH ...COMPOUND A CRYSTALLOGRAPHIC TWO-FOLD AXIS PASSES THROUGH THE TERMINAL GU WOBBLEBASE PAIR. EACH MOLECULE ABOUT THE 2-FOLD CONTRIBUTES ONE NUCLEOTIDE TO THE WOBBLE PAIR AND THE NUCLEOTIDE THAT COULD FORM THE INTRAMOLECULAR CLOSING PAIR IS DISORDERED. NOTE THAT THE OCCUPANCIES FOR THESE NUCLEOTIDES IS 0.5. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1msy.cif.gz 1msy.cif.gz | 42.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1msy.ent.gz pdb1msy.ent.gz | 30.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1msy.json.gz 1msy.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1msy_validation.pdf.gz 1msy_validation.pdf.gz | 386.5 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1msy_full_validation.pdf.gz 1msy_full_validation.pdf.gz | 387.9 KB | Display | |
| Data in XML |  1msy_validation.xml.gz 1msy_validation.xml.gz | 4.7 KB | Display | |
| Data in CIF |  1msy_validation.cif.gz 1msy_validation.cif.gz | 6.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ms/1msy https://data.pdbj.org/pub/pdb/validation_reports/ms/1msy ftp://data.pdbj.org/pub/pdb/validation_reports/ms/1msy ftp://data.pdbj.org/pub/pdb/validation_reports/ms/1msy | HTTPS FTP |
-Related structure data
| Related structure data |  483dS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: RNA chain | Mass: 8705.215 Da / Num. of mol.: 1 / Mutation: A2660U, G2661A / Source method: obtained synthetically Details: A2660U and G2661U double mutant of the SRL domain from E.coli rRNA |
|---|---|
| #2: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.15 Å3/Da / Density % sol: 42.73 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 7 Details: ammonium sulfate, potassium MOPS, magnesium chloride, manganese chloride, pH 7.0, VAPOR DIFFUSION, HANGING DROP, temperature 292K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 19 ℃ / pH: 8 / Method: vapor diffusion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.97764 Å / Beamline: 19-ID / Wavelength: 0.97764 Å |
| Detector | Type: CUSTOM-MADE / Detector: CCD / Date: Jul 6, 2000 |
| Radiation | Monochromator: Si-111 double crystal monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97764 Å / Relative weight: 1 |
| Reflection | Resolution: 1.4→40 Å / Num. all: 14372 / Num. obs: 14372 / % possible obs: 97.9 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Redundancy: 6.3 % / Rmerge(I) obs: 0.072 / Rsym value: 0.072 / Net I/σ(I): 21.8 |
| Reflection shell | Resolution: 1.4→1.42 Å / Rmerge(I) obs: 0.323 / Mean I/σ(I) obs: 2.3 / Rsym value: 0.323 / % possible all: 80 |
| Reflection | *PLUS Highest resolution: 1.4 Å / Lowest resolution: 40 Å |
| Reflection shell | *PLUS % possible obs: 80 % |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 483D Resolution: 1.41→29 Å / Num. parameters: 6236 / Num. restraintsaints: 8182 / Cross valid method: FREE R / σ(F): 0 / σ(I): 0 Details: ANISOTROPIC SCALING APPLIED BY THE METHOD OF PARKIN, MOEZZI & HOPE, J.APPL.CRYST.28(1995)53-56 ANISOTROPIC REFINEMENT REDUCED FREE R (NO CUTOFF) BY ?
| |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 2 / Occupancy sum hydrogen: 0 / Occupancy sum non hydrogen: 662.5 | |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.41→29 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||
| Software | *PLUS Name: SHELXL / Version: 97 / Classification: refinement | |||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 1.42 Å / % reflection Rfree: 10 % | |||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: s_bond_d / Dev ideal: 0.01 | |||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.458 / Rfactor Rwork: 0.278 |
 Movie
Movie Controller
Controller











 PDBj
PDBj