+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1mi0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the redesigned protein G variant NuG2 | ||||||
 Components Components | immunoglobulin-binding protein G | ||||||
 Keywords Keywords | IMMUNE SYSTEM / alpha-beta protein / redesigned beta-hairpin | ||||||
| Function / homology |  Function and homology information Function and homology informationProtein L, Ig light chain-binding / Protein L b1 domain / IgG-binding B / B domain / Ubiquitin-like (UB roll) - #10 / Ubiquitin-like (UB roll) / Roll / Alpha Beta Similarity search - Domain/homology | ||||||
| Biological species |  Finegoldia magna (bacteria) Finegoldia magna (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.85 Å MOLECULAR REPLACEMENT / Resolution: 1.85 Å | ||||||
 Authors Authors | Nauli, S. / Kuhlman, B. / Le Trong, I. / Stenkamp, R.E. / Teller, D.C. / Baker, D. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2002 Journal: Biochemistry / Year: 2002Title: Crystal structures and increased stabilization of the protein G variants with switched folding pathways NuG1 and NuG2 Authors: Nauli, S. / Kuhlman, B. / Le Trong, I. / Stenkamp, R.E. / Teller, D.C. / Baker, D. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: AUTHOR PROVIDED | ||||||
| Remark 999 | SEQUENCE THE SEQUENCE DIFFERS FROM PIR ENTRY A45063 AT RESIDUES 11-21, chain A, and residues 12-22, ...SEQUENCE THE SEQUENCE DIFFERS FROM PIR ENTRY A45063 AT RESIDUES 11-21, chain A, and residues 12-22, chain B, (PIR RESIDUES 328-384) BECAUSE THE AUTHORS REDESIGNED THE FIRST HAIRPIN. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1mi0.cif.gz 1mi0.cif.gz | 38.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1mi0.ent.gz pdb1mi0.ent.gz | 27.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1mi0.json.gz 1mi0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1mi0_validation.pdf.gz 1mi0_validation.pdf.gz | 439.6 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1mi0_full_validation.pdf.gz 1mi0_full_validation.pdf.gz | 448.3 KB | Display | |
| Data in XML |  1mi0_validation.xml.gz 1mi0_validation.xml.gz | 9.5 KB | Display | |
| Data in CIF |  1mi0_validation.cif.gz 1mi0_validation.cif.gz | 12.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mi/1mi0 https://data.pdbj.org/pub/pdb/validation_reports/mi/1mi0 ftp://data.pdbj.org/pub/pdb/validation_reports/mi/1mi0 ftp://data.pdbj.org/pub/pdb/validation_reports/mi/1mi0 | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 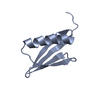
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 7356.045 Da / Num. of mol.: 2 / Fragment: Redesigned B1 domain / Mutation: D52A Source method: isolated from a genetically manipulated source Details: REDESIGNED FIRST BETA-HAIRPIN, VARIANT NUG2 / Source: (gene. exp.)  Finegoldia magna (bacteria) / Strain: ATCC 29328 / Production host: Finegoldia magna (bacteria) / Strain: ATCC 29328 / Production host:  #2: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.31 Å3/Da / Density % sol: 46.81 % | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 8 Details: ammonium sulfate, tris-hcl, pH 8, VAPOR DIFFUSION, HANGING DROP, temperature 298K | ||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 7.5 | ||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 298 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 ROTATING ANODE / Type: RIGAKU RU200 |
| Detector | Type: RIGAKU RAXIS IIC / Detector: IMAGE PLATE |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Relative weight: 1 |
| Reflection | Resolution: 1.85→19.84 Å / Num. all: 11456 / Num. obs: 11456 |
| Reflection shell | Resolution: 1.85→1.93 Å / % possible all: 83 |
| Reflection | *PLUS Num. obs: 10905 / % possible obs: 97.3 % / Rmerge(I) obs: 0.067 |
- Processing
Processing
| Software |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.85→19.84 Å MOLECULAR REPLACEMENT / Resolution: 1.85→19.84 Å
| |||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→19.84 Å
| |||||||||||||||
| Refine LS restraints |
| |||||||||||||||
| Refinement | *PLUS Lowest resolution: 15 Å / Num. reflection obs: 9780 / Rfactor Rwork: 0.26 | |||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller










 PDBj
PDBj
