+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2lhg | ||||||
|---|---|---|---|---|---|---|---|
| Title | GB98-T25I solution structure | ||||||
 Components Components | GB98 | ||||||
 Keywords Keywords | DE NOVO PROTEIN | ||||||
| Function / homology | Albumin-binding domain / Helicase, Ruva Protein; domain 3 / Orthogonal Bundle / Mainly Alpha Function and homology information Function and homology information | ||||||
| Biological species | artificial gene (others) | ||||||
| Method | SOLUTION NMR / simulated annealing | ||||||
| Model details | lowest energy, model 1 | ||||||
 Authors Authors | He, Y. / Chen, Y. / Alexander, P. / Bryan, P. / Orban, J. | ||||||
 Citation Citation |  Journal: Structure / Year: 2012 Journal: Structure / Year: 2012Title: Mutational tipping points for switching protein folds and functions. Authors: He, Y. / Chen, Y. / Alexander, P.A. / Bryan, P.N. / Orban, J. | ||||||
| History |
|
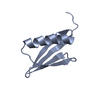
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2lhg.cif.gz 2lhg.cif.gz | 183.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2lhg.ent.gz pdb2lhg.ent.gz | 152.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2lhg.json.gz 2lhg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lh/2lhg https://data.pdbj.org/pub/pdb/validation_reports/lh/2lhg ftp://data.pdbj.org/pub/pdb/validation_reports/lh/2lhg ftp://data.pdbj.org/pub/pdb/validation_reports/lh/2lhg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2lhcC  2lhdC  2lheC C: citing same article ( |
|---|---|
| Similar structure data | |
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 6417.416 Da / Num. of mol.: 1 / Mutation: T25I Source method: isolated from a genetically manipulated source Source: (gene. exp.) artificial gene (others) / Gene: PGB98 / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Contents: 0.1-0.3 mM [U-100% 13C; U-100% 15N] GB98-T25I, 100 mM potassium phosphate, 95% H2O/5% D2O Solvent system: 95% H2O/5% D2O | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
| ||||||||||||||||||
| Sample conditions | Ionic strength: 100 / pH: 7 / Pressure: ambient / Temperature: 278 K |
-NMR measurement
| NMR spectrometer | Type: Bruker DMX / Manufacturer: Bruker / Model: DMX / Field strength: 600 MHz |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: simulated annealing / Software ordinal: 1 | ||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 3000 / Conformers submitted total number: 10 |
 Movie
Movie Controller
Controller










 PDBj
PDBj
 HSQC
HSQC