[English] 日本語
 Yorodumi
Yorodumi- PDB-1lr8: Crystal structure of Fs1, the heparin-binding domain of follistat... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1lr8 | ||||||
|---|---|---|---|---|---|---|---|
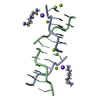
| Title | Crystal structure of Fs1, the heparin-binding domain of follistatin, complexed with the heparin analogue D-myo-inositol hexasulphate (Ins6S) | ||||||
 Components Components | Follistatin | ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / cystine-rich / D-myo-inositol hexasulphate / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationAntagonism of Activin by Follistatin / activin receptor antagonist activity / negative regulation of follicle-stimulating hormone secretion / ameloblast differentiation / positive regulation of hair follicle development / regulation of BMP signaling pathway / gamete generation / activin binding / pattern specification process / negative regulation of activin receptor signaling pathway ...Antagonism of Activin by Follistatin / activin receptor antagonist activity / negative regulation of follicle-stimulating hormone secretion / ameloblast differentiation / positive regulation of hair follicle development / regulation of BMP signaling pathway / gamete generation / activin binding / pattern specification process / negative regulation of activin receptor signaling pathway / heparan sulfate proteoglycan binding / hair follicle morphogenesis / negative regulation of epithelial cell differentiation / female gonad development / odontogenesis of dentin-containing tooth / keratinocyte proliferation / BMP signaling pathway / hematopoietic progenitor cell differentiation / skeletal system development / cellular response to growth factor stimulus / cell differentiation / nucleolus / negative regulation of transcription by RNA polymerase II / extracellular space / extracellular region / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Innis, C.A. / Hyvonen, M. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2003 Journal: J.Biol.Chem. / Year: 2003Title: Crystal Structures of the Heparan Sulfate-binding Domain of Follistatin: Insights into ligand binding. Authors: Innis, C.A. / Hyvonen, M. | ||||||
| History |
| ||||||
| Remark 600 | heterogen authors informed that the inositol ring is missing from the ligand d-myo-inositol ... heterogen authors informed that the inositol ring is missing from the ligand d-myo-inositol hexasulphate due to lack of connecting electron density. Authors state this may be due to a superimposition of inositol molecules bound in alternative ways. Although specific numbering of the ligand is present, the observed sulphate groups may correspond to one or several instances of a bound sulphate, and thus numbering of the groups is somewhat arbitrary. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1lr8.cif.gz 1lr8.cif.gz | 28.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1lr8.ent.gz pdb1lr8.ent.gz | 17.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1lr8.json.gz 1lr8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1lr8_validation.pdf.gz 1lr8_validation.pdf.gz | 810.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1lr8_full_validation.pdf.gz 1lr8_full_validation.pdf.gz | 810.8 KB | Display | |
| Data in XML |  1lr8_validation.xml.gz 1lr8_validation.xml.gz | 5.8 KB | Display | |
| Data in CIF |  1lr8_validation.cif.gz 1lr8_validation.cif.gz | 7.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lr/1lr8 https://data.pdbj.org/pub/pdb/validation_reports/lr/1lr8 ftp://data.pdbj.org/pub/pdb/validation_reports/lr/1lr8 ftp://data.pdbj.org/pub/pdb/validation_reports/lr/1lr8 | HTTPS FTP |
-Related structure data
| Related structure data |  1lr7SC  1lr9C S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 8342.812 Da / Num. of mol.: 1 / Fragment: heparin-binding domain Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Chemical | ChemComp-IHS / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.96 Å3/Da / Density % sol: 35.85 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 15-25% PEG8000, 0.2-0.6 M Magnesium acetate, 0.1 M Sodium cacodylate, pH 6.5, VAPOR DIFFUSION, HANGING DROP, temperature 289K | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 18 ℃ / pH: 8.5 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.9202 Å / Beamline: ID29 / Wavelength: 0.9202 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Jun 4, 2001 / Details: mirrors |
| Radiation | Monochromator: Si 111 CHANNEL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9202 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→38.9 Å / Num. obs: 4026 / % possible obs: 98.6 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.5 % / Biso Wilson estimate: 19.44 Å2 / Rmerge(I) obs: 0.081 |
| Reflection shell | Resolution: 2.1→2.18 Å / Rmerge(I) obs: 0.206 / % possible all: 99.7 |
| Reflection | *PLUS % possible obs: 98.6 % / Rmerge(I) obs: 0.08 |
| Reflection shell | *PLUS % possible obs: 99.7 % / Rmerge(I) obs: 0.26 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1LR7 Resolution: 2.1→38.92 Å / Cor.coef. Fo:Fc: 0.917 / Cor.coef. Fo:Fc free: 0.883 / SU B: 6.951 / SU ML: 0.189 / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.299 / ESU R Free: 0.212 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 21.635 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→38.92 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.1→2.154 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 38.9 Å / Num. reflection obs: 3801 / Rfactor Rfree: 0.251 / Rfactor Rwork: 0.221 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller








 PDBj
PDBj