[English] 日本語
 Yorodumi
Yorodumi- PDB-1i73: COMPLEX OF PRO-LEU-L-TRP PHOSPHONATE WITH THE CATALITIC DOMAIN OF... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1i73 | ||||||
|---|---|---|---|---|---|---|---|
| Title | COMPLEX OF PRO-LEU-L-TRP PHOSPHONATE WITH THE CATALITIC DOMAIN OF MATRIX METALLO PROTEINASE-8 (MET80 FORM) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / METALLOPROTEASE-INHIBITOR complex / HYDROLASE-HYDROLASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationneutrophil collagenase / tumor necrosis factor binding / positive regulation of microglial cell activation / positive regulation of tumor necrosis factor-mediated signaling pathway / positive regulation of neuroinflammatory response / Activation of Matrix Metalloproteinases / endodermal cell differentiation / Collagen degradation / collagen catabolic process / extracellular matrix disassembly ...neutrophil collagenase / tumor necrosis factor binding / positive regulation of microglial cell activation / positive regulation of tumor necrosis factor-mediated signaling pathway / positive regulation of neuroinflammatory response / Activation of Matrix Metalloproteinases / endodermal cell differentiation / Collagen degradation / collagen catabolic process / extracellular matrix disassembly / Degradation of the extracellular matrix / extracellular matrix organization / metalloendopeptidase activity / : / specific granule lumen / positive regulation of tumor necrosis factor production / tertiary granule lumen / peptidase activity / cellular response to lipopolysaccharide / endopeptidase activity / serine-type endopeptidase activity / Neutrophil degranulation / proteolysis / extracellular space / extracellular region / zinc ion binding Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.4 Å MOLECULAR REPLACEMENT / Resolution: 1.4 Å | ||||||
 Authors Authors | Gavuzzo, E. / Pochetti, G. / Mazza, F. / Gallina, C. / Gorini, B. / D'Alessio, S. / Pieper, M. / Tschesche, H. / Tucker, P.A. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2000 Journal: J.Med.Chem. / Year: 2000Title: Two crystal structures of human neutrophil collagenase, one complexed with a primed- and the other with an unprimed-side inhibitor: implications for drug design. Authors: Gavuzzo, E. / Pochetti, G. / Mazza, F. / Gallina, C. / Gorini, B. / D'Alessio, S. / Pieper, M. / Tschesche, H. / Tucker, P.A. #1:  Journal: Eur.J.Biochem. / Year: 1995 Journal: Eur.J.Biochem. / Year: 1995Title: X-RAY STRUCTURES OF HUMAN NEUTROPHIL COLLAGENASE COMPLEXED WITH PEPTIDE HYDROXAMATE AND PEPTIDE THIOL INHIBITORS. IMPLICATIONS FOR SUBSTRATE BINDING AND RATIONAL DRUG DESIGN Authors: Grams, F. / Reinemer, P. / Powers, J.C. / Kleine, T. / Pieper, M. / Tschesche, H. / Huber, R. / Bode, W. #2:  Journal: J.Med.Chem. / Year: 1999 Journal: J.Med.Chem. / Year: 1999Title: Quantitative Structure-Activity Relationship of Human Neutrophil Collagenase (Mmp8-8) Inhibitors Using Comparative Molecular Field Analysis and X-Ray Structure Analysis Authors: Matter, H. / Schwab, W. / Barbier, D. / Billen, G. / Haase, B. / Neises, B. / Schudok, M. / Thorwart, W. / Schreuder, H. / Brachvogel, V. / Loenze, P. / Weithmann, K.U. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1i73.cif.gz 1i73.cif.gz | 96.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1i73.ent.gz pdb1i73.ent.gz | 70.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1i73.json.gz 1i73.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i7/1i73 https://data.pdbj.org/pub/pdb/validation_reports/i7/1i73 ftp://data.pdbj.org/pub/pdb/validation_reports/i7/1i73 ftp://data.pdbj.org/pub/pdb/validation_reports/i7/1i73 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1i76C  1japS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 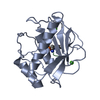
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 18111.744 Da / Num. of mol.: 1 / Fragment: RESIDUES 80-242 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  | ||||
|---|---|---|---|---|---|
| #2: Protein/peptide | Mass: 494.478 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: The three residue peptide inhibitor was chemically synthesized | ||||
| #3: Chemical | | #4: Chemical | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.14 Å3/Da / Density % sol: 42.63 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop / pH: 6 Details: PEG 6000, MES-NaOH, NaCl, CaCl2, ZnCl2, pH 6.00, VAPOR DIFFUSION, HANGING DROP, temperature 291K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 18 ℃Details: 2 micro litte of protein solution, 1 miro litter of inhibitor solution, and 5 micro litter of PEG solution | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: BW7B / Wavelength: 0.84 / Beamline: BW7B / Wavelength: 0.84 |
| Detector | Type: MAR scanner 345 mm plate / Detector: IMAGE PLATE / Date: Jul 21, 1998 |
| Radiation | Monochromator: TRIANGULAR MONOCHROMATOR / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.84 Å / Relative weight: 1 |
| Reflection | Resolution: 1.4→20 Å / Num. all: 32394 / Num. obs: 30229 / % possible obs: 93.3 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 1 / Redundancy: 3.5 % / Biso Wilson estimate: 8.5 Å2 / Rmerge(I) obs: 0.081 / Net I/σ(I): 11.5 |
| Reflection shell | Resolution: 1.4→1.42 Å / Redundancy: 2 % / Rmerge(I) obs: 0.258 / Mean I/σ(I) obs: 4.1 / % possible all: 85.6 |
| Reflection | *PLUS Num. measured all: 106034 |
| Reflection shell | *PLUS % possible obs: 85.6 % |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1JAP Resolution: 1.4→10 Å / Num. parameters: 14472 / Num. restraintsaints: 17466 / σ(F): 0 / σ(I): 0 / Stereochemistry target values: ENGH AND HUBER
| |||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 6 / Occupancy sum hydrogen: 1203 / Occupancy sum non hydrogen: 1589 | |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.4→10 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.4→10 Å
|
 Movie
Movie Controller
Controller











 PDBj
PDBj









