[English] 日本語
 Yorodumi
Yorodumi- PDB-1gc6: CRYSTAL STRUCTURE OF THE RADIXIN FERM DOMAIN COMPLEXED WITH INOSI... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1gc6 | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF THE RADIXIN FERM DOMAIN COMPLEXED WITH INOSITOL-(1,4,5)-TRIPHOSPHATE | ||||||
 Components Components | RADIXIN | ||||||
 Keywords Keywords | CELL ADHESION / CYTOSKELETON | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of actin filament bundle assembly / stereocilium base / regulation of organelle assembly / regulation of ruffle assembly / establishment of protein localization to plasma membrane / positive regulation of early endosome to late endosome transport / negative regulation of adherens junction organization / microvillus assembly / regulation of Rap protein signal transduction / Recycling pathway of L1 ...regulation of actin filament bundle assembly / stereocilium base / regulation of organelle assembly / regulation of ruffle assembly / establishment of protein localization to plasma membrane / positive regulation of early endosome to late endosome transport / negative regulation of adherens junction organization / microvillus assembly / regulation of Rap protein signal transduction / Recycling pathway of L1 / negative regulation of homotypic cell-cell adhesion / positive regulation of protein localization to early endosome / cell tip / : / regulation of postsynaptic neurotransmitter receptor diffusion trapping / barbed-end actin filament capping / stereocilium / negative regulation of cell size / cellular response to thyroid hormone stimulus / establishment of endothelial barrier / apical protein localization / cortical actin cytoskeleton / protein kinase A binding / microvillus / cleavage furrow / positive regulation of G1/S transition of mitotic cell cycle / cellular response to platelet-derived growth factor stimulus / ruffle / T-tubule / adherens junction / filopodium / establishment of protein localization / apical part of cell / positive regulation of protein catabolic process / myelin sheath / regulation of cell shape / lamellipodium / actin binding / ATPase binding / midbody / positive regulation of cell migration / apical plasma membrane / protein domain specific binding / focal adhesion / positive regulation of gene expression / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.9 Å X-RAY DIFFRACTION / Resolution: 2.9 Å | ||||||
 Authors Authors | Hamada, K. / Shimizu, T. / Matsui, T. / Tsukita, S. / Tsukita, S. / Hakoshima, T. | ||||||
 Citation Citation |  Journal: EMBO J. / Year: 2000 Journal: EMBO J. / Year: 2000Title: Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. Authors: Hamada, K. / Shimizu, T. / Matsui, T. / Tsukita, S. / Hakoshima, T. #1:  Journal: Acta Crystallogr.,Sect.D / Year: 2000 Journal: Acta Crystallogr.,Sect.D / Year: 2000Title: Crystallographic characterization of the membrane-binding domain of radixin Authors: Hamada, K. / Shimizu, T. / Matsui, T. / Tsukita, S. / Tsukita, S. / Hakoshima, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1gc6.cif.gz 1gc6.cif.gz | 72.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1gc6.ent.gz pdb1gc6.ent.gz | 54.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1gc6.json.gz 1gc6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gc/1gc6 https://data.pdbj.org/pub/pdb/validation_reports/gc/1gc6 ftp://data.pdbj.org/pub/pdb/validation_reports/gc/1gc6 ftp://data.pdbj.org/pub/pdb/validation_reports/gc/1gc6 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 35278.723 Da / Num. of mol.: 1 / Fragment: FERM DOMAIN Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
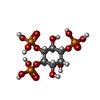
| #2: Chemical | ChemComp-I3P / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.41 Å3/Da / Density % sol: 72.08 % | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 6 Details: PEG 6000, MES, INOSITOL-(1,4,5)-TRIPHOSPHATE, pH 6.0, VAPOR DIFFUSION, HANGING DROP, temperature 277K | ||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 4 ℃ | ||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 288 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-C / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU FR-C / Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: Mar 21, 2000 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→30 Å / Num. all: 88728 / Num. obs: 14511 / % possible obs: 99.5 % / Biso Wilson estimate: 63.6 Å2 / Rmerge(I) obs: 0.081 / Net I/σ(I): 17.9 |
| Reflection shell | Resolution: 2.9→3 Å / Rmerge(I) obs: 0.485 / % possible all: 98.8 |
| Reflection shell | *PLUS % possible obs: 98.8 % / Mean I/σ(I) obs: 1.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.9→30 Å / σ(F): 2 / Stereochemistry target values: protein.para
| ||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.9→30 Å
| ||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||
| Software | *PLUS Name: CNS / Classification: refinement | ||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller














 PDBj
PDBj