[English] 日本語
 Yorodumi
Yorodumi- PDB-1ft4: PHOTOCHEMICALLY-ENHANCED BINDING OF SMALL MOLECULES TO THE TUMOR ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ft4 | ||||||
|---|---|---|---|---|---|---|---|
| Title | PHOTOCHEMICALLY-ENHANCED BINDING OF SMALL MOLECULES TO THE TUMOR NECROSIS FACTOR RECEPTOR-1 | ||||||
 Components Components | SOLUBLE TUMOR NECROSIS FACTOR RECEPTOR 1 | ||||||
 Keywords Keywords | SIGNALING PROTEIN / BINDING PROTEIN / CYTOKINE | ||||||
| Function / homology |  Function and homology information Function and homology information: / tumor necrosis factor receptor superfamily complex / pulmonary valve development / positive regulation of lipid metabolic process / aortic valve development / tumor necrosis factor receptor activity / negative regulation of extracellular matrix constituent secretion / positive regulation of apoptotic process involved in morphogenesis / : / tumor necrosis factor binding ...: / tumor necrosis factor receptor superfamily complex / pulmonary valve development / positive regulation of lipid metabolic process / aortic valve development / tumor necrosis factor receptor activity / negative regulation of extracellular matrix constituent secretion / positive regulation of apoptotic process involved in morphogenesis / : / tumor necrosis factor binding / TNFs bind their physiological receptors / TNF signaling / negative regulation of cardiac muscle hypertrophy / regulation of establishment of endothelial barrier / regulation of tumor necrosis factor-mediated signaling pathway / TNFR1-mediated ceramide production / TNFR1-induced proapoptotic signaling / prostaglandin metabolic process / Interleukin-10 signaling / extrinsic apoptotic signaling pathway via death domain receptors / positive regulation of execution phase of apoptosis / cell surface receptor signaling pathway via JAK-STAT / tumor necrosis factor-mediated signaling pathway / TNFR1-induced NF-kappa-B signaling pathway / protein localization to plasma membrane / Regulation of TNFR1 signaling / cellular response to mechanical stimulus / negative regulation of inflammatory response / cytokine-mediated signaling pathway / intrinsic apoptotic signaling pathway in response to DNA damage / positive regulation of inflammatory response / signaling receptor activity / transcription by RNA polymerase II / positive regulation of canonical NF-kappaB signal transduction / receptor complex / defense response to bacterium / membrane raft / inflammatory response / Golgi membrane / cell surface / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular region / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2.9 Å SYNCHROTRON / Resolution: 2.9 Å | ||||||
 Authors Authors | Muckelbauer, J.K. / Chang, C.-H. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2001 Journal: Proc.Natl.Acad.Sci.USA / Year: 2001Title: Photochemically enhanced binding of small molecules to the tumor necrosis factor receptor-1 inhibits the binding of TNF-alpha. Authors: Carter, P.H. / Scherle, P.A. / Muckelbauer, J.K. / Voss, M.E. / Liu, R.Q. / Thompson, L.A. / Tebben, A.J. / Solomon, K.A. / Lo, Y.C. / Li, Z. / Strzemienski, P. / Yang, G. / Falahatpisheh, N. ...Authors: Carter, P.H. / Scherle, P.A. / Muckelbauer, J.K. / Voss, M.E. / Liu, R.Q. / Thompson, L.A. / Tebben, A.J. / Solomon, K.A. / Lo, Y.C. / Li, Z. / Strzemienski, P. / Yang, G. / Falahatpisheh, N. / Xu, M. / Wu, Z. / Farrow, N.A. / Ramnarayan, K. / Wang, J. / Rideout, D. / Yalamoori, V. / Domaille, P. / Underwood, D.J. / Trzaskos, J.M. / Friedman, S.M. / Newton, R.C. / Decicco, C.P. / Muckelbauer, J.A. #1:  Journal: J.Biol.Chem. / Year: 1995 Journal: J.Biol.Chem. / Year: 1995Title: Crystallographic Evidence for Dimerization of Unliganded Tumor Necrosis Factor Receptor Authors: Naismith, J.H. / Devine, T.Q. / Brandhuber, B.J. / Sprang, S.R. #2:  Journal: Cell(Cambridge,Mass.) / Year: 1993 Journal: Cell(Cambridge,Mass.) / Year: 1993Title: Crystal Structure of the Soluble Human 55 Kd Tnf Receptor-Human Tnfb Complex: Implication for Tnf Receptor Activation Authors: Banner, D.W. / D'Arcy, A. / Janes, W. / Gentz, W. / Schoenfeld, H.-J. / Broger, C. / Loetscher, H. / Lesslauer, W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ft4.cif.gz 1ft4.cif.gz | 70 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ft4.ent.gz pdb1ft4.ent.gz | 52.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ft4.json.gz 1ft4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1ft4_validation.pdf.gz 1ft4_validation.pdf.gz | 482.4 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1ft4_full_validation.pdf.gz 1ft4_full_validation.pdf.gz | 500.1 KB | Display | |
| Data in XML |  1ft4_validation.xml.gz 1ft4_validation.xml.gz | 9.8 KB | Display | |
| Data in CIF |  1ft4_validation.cif.gz 1ft4_validation.cif.gz | 13.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ft/1ft4 https://data.pdbj.org/pub/pdb/validation_reports/ft/1ft4 ftp://data.pdbj.org/pub/pdb/validation_reports/ft/1ft4 ftp://data.pdbj.org/pub/pdb/validation_reports/ft/1ft4 | HTTPS FTP |
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
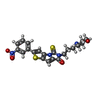
| #1: Protein | Mass: 18335.830 Da / Num. of mol.: 2 Fragment: 55 KD EXTRACELLULAR DOMAIN (MET PLUS RESIDUES 12-272) Source method: isolated from a genetically manipulated source Details: N OF ALA 62, CHAIN A IS BOUND TO LIGAND 705 / Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  #2: Chemical | ChemComp-703 / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.98 Å3/Da / Density % sol: 58.67 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 297 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: MPD, Ammonium Acetate Tris Buffer, pH 8.5, VAPOR DIFFUSION, SITTING DROP at 297K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 293 K / Details: Rodseth, L.E., (1994) J.Mol.Biol., 239, 332. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 5ID-B / Wavelength: 1 / Beamline: 5ID-B / Wavelength: 1 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Feb 13, 2000 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→35 Å / Num. obs: 9191 / % possible obs: 88 % / Rmerge(I) obs: 0.093 / Net I/σ(I): 17 |
- Processing
Processing
| Software |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.9→10 Å / σ(F): 2 /
| |||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.9→10 Å
| |||||||||||||||
| Refine LS restraints |
| |||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | |||||||||||||||
| Refinement | *PLUS Lowest resolution: 10 Å / σ(F): 2 / Rfactor obs: 0.274 | |||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_deg / Dev ideal: 3.1 |
 Movie
Movie Controller
Controller











 PDBj
PDBj