[English] 日本語
 Yorodumi
Yorodumi- PDB-1c9d: CRYSTAL STRUCTURE OF THE COMPLEX OF BACTERIAL TRYPTOPHAN SYNTHASE... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1c9d | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF THE COMPLEX OF BACTERIAL TRYPTOPHAN SYNTHASE WITH THE TRANSITION STATE ANALOGUE INHIBITOR 4-(2-HYDROXY-4-FLUOROPHENYLTHIO)-BUTYLPHOSPHONIC ACID | ||||||
 Components Components | (TRYPTOPHAN SYNTHASE ...) x 2 | ||||||
 Keywords Keywords | LYASE / 8-FOLD ALPHA-BETA BARREL / ENZYME-INHIBITOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationtryptophan synthase / tryptophan synthase activity / L-tryptophan biosynthetic process / identical protein binding / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Salmonella typhimurium (bacteria) Salmonella typhimurium (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.3 Å X-RAY DIFFRACTION / Resolution: 2.3 Å | ||||||
 Authors Authors | Lolis, E. / Sachpatzidis, A. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 1999 Journal: Biochemistry / Year: 1999Title: Crystallographic studies of phosphonate-based alpha-reaction transition-state analogues complexed to tryptophan synthase. Authors: Sachpatzidis, A. / Dealwis, C. / Lubetsky, J.B. / Liang, P.H. / Anderson, K.S. / Lolis, E. #1:  Journal: J.Biol.Chem. / Year: 1988 Journal: J.Biol.Chem. / Year: 1988Title: Three-Dimensional Structure of the Tryptophan Synthase Alpha 2 Beta 2 Multienzyme Complex from Salmonella typhimurium Authors: Hyde, C.C. / Ahmed, S.A. / Padlan, E.A. / Miles, E.W. / Davies, D.R. #2:  Journal: Biochemistry / Year: 1998 Journal: Biochemistry / Year: 1998Title: Loop Closure and Intersubunit Communication in Tryptophan Synthase Authors: Schneider, T.R. / Gerhardt, E. / Lee, M. / Liang, P.H. / Anderson, K.S. / Schlichting, I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1c9d.cif.gz 1c9d.cif.gz | 137.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1c9d.ent.gz pdb1c9d.ent.gz | 106.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1c9d.json.gz 1c9d.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1c9d_validation.pdf.gz 1c9d_validation.pdf.gz | 766.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1c9d_full_validation.pdf.gz 1c9d_full_validation.pdf.gz | 779.9 KB | Display | |
| Data in XML |  1c9d_validation.xml.gz 1c9d_validation.xml.gz | 27.3 KB | Display | |
| Data in CIF |  1c9d_validation.cif.gz 1c9d_validation.cif.gz | 38.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c9/1c9d https://data.pdbj.org/pub/pdb/validation_reports/c9/1c9d ftp://data.pdbj.org/pub/pdb/validation_reports/c9/1c9d ftp://data.pdbj.org/pub/pdb/validation_reports/c9/1c9d | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-TRYPTOPHAN SYNTHASE ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 28698.797 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Salmonella typhimurium (bacteria) / Production host: Salmonella typhimurium (bacteria) / Production host:  |
|---|---|
| #2: Protein | Mass: 42988.996 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Salmonella typhimurium (bacteria) / Production host: Salmonella typhimurium (bacteria) / Production host:  |
-Non-polymers , 4 types, 191 molecules 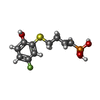






| #3: Chemical | ChemComp-HF1 / |
|---|---|
| #4: Chemical | ChemComp-NA / |
| #5: Chemical | ChemComp-PLP / |
| #6: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.63 Å3/Da / Density % sol: 53.17 % | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 7.8 Details: 12% PEG 4000, 0.75MM SPERMINE, 50MM SODIUM BICINE, 1MM EDTA, 5MM DTT, pH 7.8, VAPOR DIFFUSION, HANGING DROP, temperature 295K | |||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion | |||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 140 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS IIC / Detector: IMAGE PLATE / Date: Jan 15, 1997 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→45.9 Å / Num. all: 31780 / Num. obs: 31780 / % possible obs: 94.6 % / Redundancy: 2.1 % / Biso Wilson estimate: 11.03 Å2 / Rmerge(I) obs: 0.073 / Net I/σ(I): 14.26 |
| Reflection shell | Resolution: 2.3→2.4 Å / Redundancy: 2.1 % / Rmerge(I) obs: 0.191 / % possible all: 81.2 |
| Reflection | *PLUS % possible obs: 95.2 % / Num. measured all: 95281 |
| Reflection shell | *PLUS % possible obs: 79.6 % / Rmerge(I) obs: 0.173 / Mean I/σ(I) obs: 4.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.3→30 Å / σ(F): 2 / Stereochemistry target values: ENGH & HUBER Details: RESTRAINED LEAST SQUARES REFINEMENT. CONJUGATE GRADIENT MINIMIZATION AND SIMULATED ANNEALING PROTOCOLS IMPLEMENTED IN XPLOR.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.851 / Classification: refinement X-PLOR / Version: 3.851 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.3 Å / Lowest resolution: 30 Å / σ(F): 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller


























 PDBj
PDBj



