[English] 日本語
 Yorodumi
Yorodumi- PDB-1brb: CRYSTAL STRUCTURES OF RAT ANIONIC TRYPSIN COMPLEXED WITH THE PROT... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1brb | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURES OF RAT ANIONIC TRYPSIN COMPLEXED WITH THE PROTEIN INHIBITORS APPI AND BPTI | ||||||
 Components Components |
| ||||||
 Keywords Keywords | COMPLEX(PROTEINASE/INHIBITOR) / COMPLEX(PROTEINASE-INHIBITOR) / COMPLEX(PROTEINASE-INHIBITOR) complex | ||||||
| Function / homology |  Function and homology information Function and homology informationAntimicrobial peptides / Alpha-defensins / Activation of Matrix Metalloproteinases / sulfate binding / negative regulation of platelet aggregation / potassium channel inhibitor activity / zymogen binding / Neutrophil degranulation / molecular function inhibitor activity / negative regulation of thrombin-activated receptor signaling pathway ...Antimicrobial peptides / Alpha-defensins / Activation of Matrix Metalloproteinases / sulfate binding / negative regulation of platelet aggregation / potassium channel inhibitor activity / zymogen binding / Neutrophil degranulation / molecular function inhibitor activity / negative regulation of thrombin-activated receptor signaling pathway / collagen catabolic process / trypsin / serine protease inhibitor complex / digestion / response to nutrient / serine-type endopeptidase inhibitor activity / protease binding / serine-type endopeptidase activity / calcium ion binding / proteolysis / extracellular space / extracellular region Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.1 Å X-RAY DIFFRACTION / Resolution: 2.1 Å | ||||||
 Authors Authors | Perona, J.J. / Fletterick, R.J. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1993 Journal: J.Mol.Biol. / Year: 1993Title: Crystal structures of rat anionic trypsin complexed with the protein inhibitors APPI and BPTI. Authors: Perona, J.J. / Tsu, C.A. / Craik, C.S. / Fletterick, R.J. #1:  Journal: J.Mol.Biol. / Year: 1993 Journal: J.Mol.Biol. / Year: 1993Title: Relocating a Negative Charge in the Binding Pocket of Trypsin Authors: Perona, J.J. / Tsu, C.A. / Mcgrath, M.E. / Craik, C.S. / Fletterick, R.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
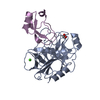
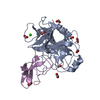
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1brb.cif.gz 1brb.cif.gz | 68.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1brb.ent.gz pdb1brb.ent.gz | 49.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1brb.json.gz 1brb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/br/1brb https://data.pdbj.org/pub/pdb/validation_reports/br/1brb ftp://data.pdbj.org/pub/pdb/validation_reports/br/1brb ftp://data.pdbj.org/pub/pdb/validation_reports/br/1brb | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 23814.838 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
|---|---|
| #2: Protein | Mass: 6407.332 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source References: UniProt: P00974 |
| #3: Water | ChemComp-HOH / |
| Compound details | THE BPTI INHIBITOR IS THE VARIANT C5A/C55A PRODUCED BY RECOMBINANT DNA METHODOLOGIES AND EXPRESSED ...THE BPTI INHIBITOR IS THE VARIANT C5A/C55A PRODUCED BY RECOMBINAN |
| Has protein modification | Y |
| Sequence details | SEQUENCE ADVISORY NOTICE: SEQUENCE FOR TRYPSIN IN THIS STRUCTURE WAS TAKEN FROM GENEMBL WHICH ...SEQUENCE ADVISORY NOTICE: SEQUENCE FOR TRYPSIN IN THIS STRUCTURE WAS TAKEN FROM GENEMBL WHICH DIFFERS FROM SWISSPROT SEQUENCE AT POSITIONS 61 AND 65. SEQUENCE ADVISORY NOTICE DIFFERENCE |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.55 Å3/Da / Density % sol: 51.83 % | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 4 ℃ / Method: vapor diffusion, hanging drop / PH range low: 7 / PH range high: 6.5 | |||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 2.1 Å / Num. obs: 17414 / % possible obs: 93 % / Num. measured all: 66494 / Rmerge(I) obs: 0.097 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Rfactor Rwork: 0.198 / Rfactor obs: 0.198 / Highest resolution: 2.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 2.1 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 6 Å / Rfactor obs: 0.198 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_d / Dev ideal: 2.8 |
 Movie
Movie Controller
Controller










 PDBj
PDBj









