[English] 日本語
 Yorodumi
Yorodumi- PDB-1bm2: GRB2-SH2 DOMAIN IN COMPLEX WITH CYCLO-[N-ALPHA-ACETYL-L-THI ALYSY... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1bm2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | GRB2-SH2 DOMAIN IN COMPLEX WITH CYCLO-[N-ALPHA-ACETYL-L-THI ALYSYL-O-PHOSPHOTYROSYL-VALYL-ASPARAGYL-VALYL-PROLYL] (PKF273-791) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / SH2 DOMAIN / SIGNAL TRANSDUCTION / ADAPTOR PROTEIN / RAS PATHWAY / CYCLIC PEPTIDE / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationguanyl-nucleotide exchange factor adaptor activity / Grb2-EGFR complex / branching involved in labyrinthine layer morphogenesis / STAT5 Activation / Co-inhibition by BTLA / COP9 signalosome / neurotrophin TRKA receptor binding / Activated NTRK2 signals through PI3K / transmembrane receptor protein tyrosine kinase adaptor activity / MET receptor recycling ...guanyl-nucleotide exchange factor adaptor activity / Grb2-EGFR complex / branching involved in labyrinthine layer morphogenesis / STAT5 Activation / Co-inhibition by BTLA / COP9 signalosome / neurotrophin TRKA receptor binding / Activated NTRK2 signals through PI3K / transmembrane receptor protein tyrosine kinase adaptor activity / MET receptor recycling / Signaling by cytosolic FGFR1 fusion mutants / Interleukin-15 signaling / MET activates PTPN11 / negative regulation of natural killer cell mediated cytotoxicity / MET activates RAP1 and RAC1 / vesicle membrane / Signaling by LTK / MET activates PI3K/AKT signaling / CD28 dependent Vav1 pathway / Signal regulatory protein family interactions / epidermal growth factor receptor binding / Regulation of KIT signaling / PI-3K cascade:FGFR3 / natural killer cell mediated cytotoxicity / STAT5 activation downstream of FLT3 ITD mutants / PI-3K cascade:FGFR2 / PI-3K cascade:FGFR4 / PI-3K cascade:FGFR1 / positive regulation of actin filament polymerization / endodermal cell differentiation / GRB2:SOS provides linkage to MAPK signaling for Integrins / RHOU GTPase cycle / regulation of MAPK cascade / RET signaling / PI3K events in ERBB2 signaling / insulin receptor substrate binding / Interleukin-3, Interleukin-5 and GM-CSF signaling / PI3K Cascade / SOS-mediated signalling / Activated NTRK3 signals through RAS / Activated NTRK2 signals through RAS / signal transduction in response to DNA damage / fibroblast growth factor receptor signaling pathway / SHC1 events in ERBB4 signaling / RHO GTPases Activate WASPs and WAVEs / Role of LAT2/NTAL/LAB on calcium mobilization / Interleukin receptor SHC signaling / Signalling to RAS / GAB1 signalosome / Signal attenuation / Activated NTRK2 signals through FRS2 and FRS3 / SHC-related events triggered by IGF1R / Schwann cell development / SHC-mediated cascade:FGFR3 / MET activates RAS signaling / SHC-mediated cascade:FGFR2 / Signaling by PDGFRA transmembrane, juxtamembrane and kinase domain mutants / Signaling by PDGFRA extracellular domain mutants / SHC-mediated cascade:FGFR4 / Erythropoietin activates RAS / Signaling by FGFR4 in disease / SHC-mediated cascade:FGFR1 / FRS-mediated FGFR3 signaling / Signaling by CSF3 (G-CSF) / Signaling by FLT3 ITD and TKD mutants / ephrin receptor binding / FRS-mediated FGFR2 signaling / FRS-mediated FGFR4 signaling / InlB-mediated entry of Listeria monocytogenes into host cell / Signaling by FGFR3 in disease / FRS-mediated FGFR1 signaling / Tie2 Signaling / Signaling by FGFR2 in disease / phosphotyrosine residue binding / GRB2 events in EGFR signaling / SHC1 events in EGFR signaling / myelination / Signaling by FLT3 fusion proteins / FLT3 Signaling / EGFR Transactivation by Gastrin / Signaling by FGFR1 in disease / FCERI mediated Ca+2 mobilization / NCAM signaling for neurite out-growth / GRB2 events in ERBB2 signaling / cellular response to ionizing radiation / Downstream signal transduction / SHC1 events in ERBB2 signaling / Insulin receptor signalling cascade / insulin-like growth factor receptor signaling pathway / Regulation of signaling by CBL / Negative regulation of FGFR3 signaling / Antigen activates B Cell Receptor (BCR) leading to generation of second messengers / Constitutive Signaling by Overexpressed ERBB2 / Signaling by phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants / T cell activation / Negative regulation of FGFR2 signaling / Negative regulation of FGFR4 signaling / Negative regulation of FGFR1 signaling / EGFR downregulation / Spry regulation of FGF signaling Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Rondeau, J.M. / Zurini, M. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 1999 Journal: J.Med.Chem. / Year: 1999Title: Structural and conformational requirements for high-affinity binding to the SH2 domain of Grb2(1). Authors: Ettmayer, P. / France, D. / Gounarides, J. / Jarosinski, M. / Martin, M.S. / Rondeau, J.M. / Sabio, M. / Topiol, S. / Weidmann, B. / Zurini, M. / Bair, K.W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1bm2.cif.gz 1bm2.cif.gz | 37.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1bm2.ent.gz pdb1bm2.ent.gz | 24.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1bm2.json.gz 1bm2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1bm2_validation.pdf.gz 1bm2_validation.pdf.gz | 426.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1bm2_full_validation.pdf.gz 1bm2_full_validation.pdf.gz | 427.6 KB | Display | |
| Data in XML |  1bm2_validation.xml.gz 1bm2_validation.xml.gz | 7.3 KB | Display | |
| Data in CIF |  1bm2_validation.cif.gz 1bm2_validation.cif.gz | 9.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bm/1bm2 https://data.pdbj.org/pub/pdb/validation_reports/bm/1bm2 ftp://data.pdbj.org/pub/pdb/validation_reports/bm/1bm2 ftp://data.pdbj.org/pub/pdb/validation_reports/bm/1bm2 | HTTPS FTP |
-Related structure data
| Related structure data | 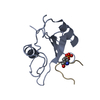 1bmbSC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 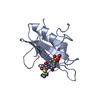
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| 2 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 13577.362 Da / Num. of mol.: 1 / Fragment: SH2 DOMAIN Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cellular location: CYTOPLASM / Gene: GRB2 / Cellular location (production host): CYTOPLASM / Production host: Homo sapiens (human) / Cellular location: CYTOPLASM / Gene: GRB2 / Cellular location (production host): CYTOPLASM / Production host:  |
|---|---|
| #2: Protein/peptide | Mass: 842.896 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: ACE: N-TERMINAL ACETYL GROUP PTR: L-PHOSPHOTYROSINE |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.4 Å3/Da / Density % sol: 49 % | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 7 Details: PROTEIN (15MG/ML IN 100MM SODIUM CHLORIDE, 0.02% SODIUM AZIDE) WAS CRYSTALLIZED FROM 30% SATURATED AMMONIUM SULFATE, 100MM SODIUM HEPES PH 7.0, IN PRESENCE OF A 2-FOLD EXCESS OF LIGAND | |||||||||||||||||||||||||||||||||||
| Crystal | *PLUS | |||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop | |||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM1A / Wavelength: 0.873 / Beamline: BM1A / Wavelength: 0.873 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Jan 19, 1997 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.873 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→8 Å / Num. obs: 7262 / % possible obs: 95.6 % / Observed criterion σ(I): 0 / Redundancy: 4.6 % / Biso Wilson estimate: 15.2 Å2 / Rmerge(I) obs: 0.044 / Net I/σ(I): 15.3 |
| Reflection shell | Resolution: 2.1→2.17 Å / Redundancy: 4.5 % / Rmerge(I) obs: 0.199 / Mean I/σ(I) obs: 4.1 / % possible all: 96.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1BMB Resolution: 2.1→8 Å / Rfactor Rfree error: 0.009 / Data cutoff high absF: 1000000 / Data cutoff low absF: 0.1 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27.3 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.1→2.23 Å / Rfactor Rfree error: 0.034 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.1 / Classification: refinement X-PLOR / Version: 3.1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS σ(F): 2 / % reflection Rfree: 10.3 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 27.3 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.358 / % reflection Rfree: 9.4 % / Rfactor Rwork: 0.281 |
 Movie
Movie Controller
Controller










 PDBj
PDBj
























