[English] 日本語
 Yorodumi
Yorodumi- EMDB-9383: Encapsulin iron storage compartment from Quasibacillus thermotolerans -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9383 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Encapsulin iron storage compartment from Quasibacillus thermotolerans | |||||||||
 Map data Map data | Final EM map filtered to 3.85A with applied sharpening B-factor of -151 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | encapsulin / iron storage / IMEF / icosahedral / METAL TRANSPORT | |||||||||
| Function / homology | Type 1 encapsulin shell protein / Encapsulating protein for peroxidase / : / encapsulin nanocompartment / iron ion transport / intracellular iron ion homeostasis / Type 1 encapsulin shell protein Function and homology information Function and homology information | |||||||||
| Biological species |   | |||||||||
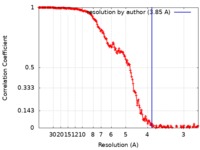
| Method | single particle reconstruction / cryo EM / Resolution: 3.85 Å | |||||||||
 Authors Authors | Orlando BJ / Giessen TW | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: Large protein organelles form a new iron sequestration system with high storage capacity. Authors: Tobias W Giessen / Benjamin J Orlando / Andrew A Verdegaal / Melissa G Chambers / Jules Gardener / David C Bell / Gabriel Birrane / Maofu Liao / Pamela A Silver /  Abstract: Iron storage proteins are essential for cellular iron homeostasis and redox balance. Ferritin proteins are the major storage units for bioavailable forms of iron. Some organisms lack ferritins, and ...Iron storage proteins are essential for cellular iron homeostasis and redox balance. Ferritin proteins are the major storage units for bioavailable forms of iron. Some organisms lack ferritins, and it is not known how they store iron. Encapsulins, a class of protein-based organelles, have recently been implicated in microbial iron and redox metabolism. Here, we report the structural and mechanistic characterization of a 42 nm two-component encapsulin-based iron storage compartment from . Using cryo-electron microscopy and x-ray crystallography, we reveal the assembly principles of a thermostable T = 4 shell topology and its catalytic ferroxidase cargo and show interactions underlying cargo-shell co-assembly. This compartment has an exceptionally large iron storage capacity storing over 23,000 iron atoms. Our results reveal a new approach for survival in diverse habitats with limited or fluctuating iron availability via an iron storage system able to store 10 to 20 times more iron than ferritin. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9383.map.gz emd_9383.map.gz | 386.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9383-v30.xml emd-9383-v30.xml emd-9383.xml emd-9383.xml | 13.2 KB 13.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9383_fsc.xml emd_9383_fsc.xml | 19.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_9383.png emd_9383.png | 262.1 KB | ||
| Filedesc metadata |  emd-9383.cif.gz emd-9383.cif.gz | 5.7 KB | ||
| Others |  emd_9383_additional.map.gz emd_9383_additional.map.gz | 335.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9383 http://ftp.pdbj.org/pub/emdb/structures/EMD-9383 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9383 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9383 | HTTPS FTP |
-Related structure data
| Related structure data |  6nj8MC  6n63C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9383.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9383.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final EM map filtered to 3.85A with applied sharpening B-factor of -151 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.365 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
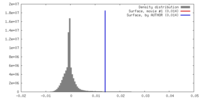
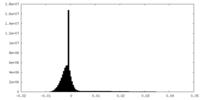
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Final unfiltered EM map
| File | emd_9383_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final unfiltered EM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
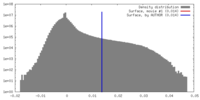
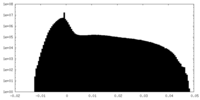
| Density Histograms |
- Sample components
Sample components
-Entire : Encapsulin iron storage compartment from Quasibacillus thermotolerans
| Entire | Name: Encapsulin iron storage compartment from Quasibacillus thermotolerans |
|---|---|
| Components |
|
-Supramolecule #1: Encapsulin iron storage compartment from Quasibacillus thermotolerans
| Supramolecule | Name: Encapsulin iron storage compartment from Quasibacillus thermotolerans type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 9.6 MDa |
-Macromolecule #1: Encapsulating protein for a DyP-type peroxidase
| Macromolecule | Name: Encapsulating protein for a DyP-type peroxidase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 32.239459 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNKSQLYPDS PLTDQDFNQL DQTVIEAARR QLVGRRFIEL YGPLGRGMQS VFNDIFMESH EAKMDFQGSF DTEVESSRRV NYTIPMLYK DFVLYWRDLE QSKALDIPID FSVAANAARD VAFLEDQMIF HGSKEFDIPG LMNVKGRLTH LIGNWYESGN A FQDIVEAR ...String: MNKSQLYPDS PLTDQDFNQL DQTVIEAARR QLVGRRFIEL YGPLGRGMQS VFNDIFMESH EAKMDFQGSF DTEVESSRRV NYTIPMLYK DFVLYWRDLE QSKALDIPID FSVAANAARD VAFLEDQMIF HGSKEFDIPG LMNVKGRLTH LIGNWYESGN A FQDIVEAR NKLLEMNHNG PYALVLSPEL YSLLHRVHKD TNVLEIEHVR ELITAGVFQS PVLKGKSGVI VNTGRNNLDL AI SEDFETA YLGEEGMNHP FRVYETVVLR IKRPAAICTL IDPEE UniProtKB: Type 1 encapsulin shell protein |
-Macromolecule #2: targeting peptide
| Macromolecule | Name: targeting peptide / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 716.824 Da |
| Recombinant expression | Organism:  |
| Sequence | String: TVGSLIQ |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: Quantifoil R1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Instrument: GATAN CRYOPLUNGE 3 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number real images: 601 / Average exposure time: 7.2 sec. / Average electron dose: 44.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)