+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9248 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of full-length IP3R1 channel in Apo-state | |||||||||||||||||||||
 Map data Map data | Cryo-EM density map of IP3R1 in apo-state | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
| Biological species |  | |||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||||||||||||||
 Authors Authors | Fan G / Baker MR / Wang Z / Seryshev AB / Ludtke SJ / Baker ML / Serysheva II | |||||||||||||||||||||
| Funding support |  United States, 6 items United States, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Cell Res / Year: 2018 Journal: Cell Res / Year: 2018Title: Cryo-EM reveals ligand induced allostery underlying InsPR channel gating. Authors: Guizhen Fan / Mariah R Baker / Zhao Wang / Alexander B Seryshev / Steven J Ludtke / Matthew L Baker / Irina I Serysheva /  Abstract: Inositol-1,4,5-trisphosphate receptors (InsPRs) are cation channels that mobilize Ca from intracellular stores in response to a wide range of cellular stimuli. The paradigm of InsPR activation is the ...Inositol-1,4,5-trisphosphate receptors (InsPRs) are cation channels that mobilize Ca from intracellular stores in response to a wide range of cellular stimuli. The paradigm of InsPR activation is the coupled interplay between binding of InsP and Ca that switches the ion conduction pathway between closed and open states to enable the passage of Ca through the channel. However, the molecular mechanism of how the receptor senses and decodes ligand-binding signals into gating motion remains unknown. Here, we present the electron cryo-microscopy structure of InsPR1 from rat cerebellum determined to 4.1 Å resolution in the presence of activating concentrations of Ca and adenophostin A (AdA), a structural mimetic of InsP and the most potent known agonist of the channel. Comparison with the 3.9 Å-resolution structure of InsPR1 in the Apo-state, also reported herein, reveals the binding arrangement of AdA in the tetrameric channel assembly and striking ligand-induced conformational rearrangements within cytoplasmic domains coupled to the dilation of a hydrophobic constriction at the gate. Together, our results provide critical insights into the mechanistic principles by which ligand-binding allosterically gates InsPR channel. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9248.map.gz emd_9248.map.gz | 28.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9248-v30.xml emd-9248-v30.xml emd-9248.xml emd-9248.xml | 16.4 KB 16.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9248.png emd_9248.png | 197.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9248 http://ftp.pdbj.org/pub/emdb/structures/EMD-9248 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9248 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9248 | HTTPS FTP |
-Related structure data
| Related structure data |  9243C  9244C  9245C  9246C  9247C  6mu1C  6mu2C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_9248.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9248.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM density map of IP3R1 in apo-state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.26 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
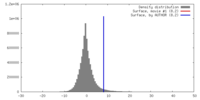
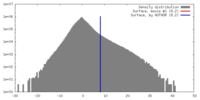
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Inositol 1,4,5-trisphosphate receptor
| Entire | Name: Inositol 1,4,5-trisphosphate receptor |
|---|---|
| Components |
|
-Supramolecule #1: Inositol 1,4,5-trisphosphate receptor
| Supramolecule | Name: Inositol 1,4,5-trisphosphate receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: tetrameric assembly |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 1.3 MDa |
-Macromolecule #1: Inositol 1,4,5-trisphosphate receptor
| Macromolecule | Name: Inositol 1,4,5-trisphosphate receptor / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MSDKMSSFLH IGDICSLYAE GSTNGFISTL GLVDDRCVVQ PEAGDLNNPP KKFRDCLFKL CPMNRYSAQK QFWKAAKPG ANSTTDAVLL NKLHHAADLE KKQNETENRK LLGTVIQYGN VIQLLHLKSN KYLTVNKRLP A LLEKNAMR VTLDEAGNEG SWFYIQPFYK ...String: MSDKMSSFLH IGDICSLYAE GSTNGFISTL GLVDDRCVVQ PEAGDLNNPP KKFRDCLFKL CPMNRYSAQK QFWKAAKPG ANSTTDAVLL NKLHHAADLE KKQNETENRK LLGTVIQYGN VIQLLHLKSN KYLTVNKRLP A LLEKNAMR VTLDEAGNEG SWFYIQPFYK LRSIGDSVVI GDKVVLNPVN AGQPLHASSH QLVDNPGCNE VN SVNCNTS WKIVLFMKWS DNKDDILKGG DVVRLFHAEQ EKFLTCDEHR KKQHVFLRTT GRQSATSATS SKA LWEVEV VQHDPCRGGA GYWNSLFRFK HLATGHYLAA EVDPDFEEEC LEFQPSVDPD QDASRSRLRN AQEK MVYSL VSVPEGNDIS SIFELDPTTL RGGDSLVPRN SYVRLRHLCT NTWVHSTNIP IDKEEEKPVM LKIGT SPLK EDKEAFAIVP VSPAEVRDLD FANDASKVLG SIAGKLEKGT ITQNERRSVT KLLEDLVYFV TGGTNS GQD VLEVVFSKPN RERQKLMREQ NILKQIFKLL QAPFTDCGDG PMLRLEELGD QRHAPFRHIC RLCYRVL RH SQQDYRKNQE YIAKQFGFMQ KQIGYDVLAE DTITALLHNN RKLLEKHITA AEIDTFVSLV RKNREPRF L DYLSDLCVSM NKSIPVTQEL ICKAVLNPTN ADILIETKLV LSRFEFEGVS TGENALEAGE DEEEVWLFW RDSNKEIRSK SVRELAQDAK EGQKEDRDVL SYYRYQLNLF ARMCLDRQYL AINEISGQLD VDLILRCMSD ENLPYDLRA SFCRLMLHMH VDRDPQEQVT PVKYARLWSE IPSEIAIDDY DSSGASKDEI KERFAQTMEF V EEYLRDVV CQRFPFSDKE KNKLTFEVVN LARNLIYFGF YNFSDLLRLT KILLAILDCV HVTTIFPISK MT KGEENKG SNVMRSIHGV GELMTQVVLR GGGFLPMTPM AAAPEGNVKQ AEPEKEDIMV MDTKLKIIEI LQF ILNVRL DYRISCLLCI FKREFDESNS QSSETSSGNS SQEGPSNVPG ALDFEHIEEQ AEGIFGGSEE NTPL DLDDH GGRTFLRVLL HLTMHDYPPL VSGALQLLFR HFSQRQEVLQ AFKQVQLLVT SQDVDNYKQI KQDLD QLRS IVEKSELWVY KGQGPDEPMD GASGENEHKK TEEGTSKPLK HESTSSYNYR VVKEILIRLS KLCVQE SAS VRKSRKQQQR LLRNMGAHAV VLELLQIPYE KAEDTKMQEI MRLAHEFLQN FCAGNQQNQA LLHKHIN LF LNPGILEAVT MQHIFMNNFQ LCSEINERVV QHFVHCIETH GRNVQYIKFL QTIVKAEGKF IKKCQDMV M AELVNSGEDV LVFYNDRASF QTLIQMMRSE RDRMDENSPL FMYHIHLVEL LAVCTEGKNV YTEIKCNSL LPLDDIVRVV THEDCIPEVK IAYINFLNHC YVDTEVEMKE IYTSNHMWKL FENFLVDICR ACNNTSDRKH ADSVLEKYV TEIVMSIVTT FFSSPFSDQS TTLQTRQPVF VQLLQGVFRV YHCNWLMPSQ KASVESCIRV L SDVAKSRA IAIPVDLDSQ VNNLFLKSHN IVQKTAMNWR LSARNAARRD SVLAASRDYR NIIERLQDIV SA LEDRLRP LVQAELSVLV DVLHRPELLF PENTDARRKC ESGGFICKLI KHTKQLLEEN EEKLCIKVLQ TLR EMMTKD RGYGEKQISI DELENAELPQ PPEAENSTEQ ELEPSPPLRQ LEDHKRGEAL RQILVNRYYG NIRP SGRRE SLTSFGNGPL SPGGPSKPGG GGGGPGSGST SRGEMSLAEV QCHLDKEGAS NLVIDLIMNA SSDRV FHES ILLAIALLEG GNTTIQHSFF CRLTEDKKSE KFFKVFYDRM KVAQQEIKAT VTVNTSDLGN KKKDDE VDR DAPSRKKAKE PTTQITEEVR DQLLEASAAT RKAFTTFRRE ADPDDHYQSG EGTQATTDKA KDDLEMS AV ITIMQPILRF LQLLCENHNR DLQNFLRCQN NKTNYNLVCE TLQFLDCICG STTGGLGLLG LYINEKNV A LINQTLESLT EYCQGPCHEN QNCIATHESN GIDIITALIL NDINPLGKKR MDLVLELKNN ASKLLLAIM ESRHDSENAE RILYNMRPKE LVEVIKKAYM QGEVEFEDGE NGEDGAASPR NVGHNIYILA HQLARHNKEL QTMLKPGGQ VDGDEALEFY AKHTAQIEIV RLDRTMEQIV FPVPSICEFL TKESKLRIYY TTERDEQGSK I NDFFLRSE DLFNEMNWQK KLRAQPVLYW CARNMSFWSS ISFNLAVLMN LLVAFFYPFK GVRGGTLEPH WS GLLWTAM LISLAIVIAL PKPHGIRALI ASTILRLIFS VGLQPTLFLL GAFNVCNKII FLMSFVGNCG TFT RGYRAM VLDVEFLYHL LYLLICAMGL FVHEFFYSLL LFDLVYREET LLNVIKSVTR NGRPIILTAA LALI LVYLF SIVGYLFFKD DFILEVDRLP NETAGPETGE SLANDFLYSD VCRVETGENC TSPAPKEELL PVEET EQDK EHTCETLLMC IVTVLSHGLR SGGGVGDVLR KPSKEEPLFA ARVIYDLLFF FMVIIIVLNL IFGVII DTF ADLRSEKQKK EEILKTTCFI CGLERDKFDN KTVTFEEHIK EEHNMWHYLC FIVLVKVKDS TEYTGPE SY VAEMIRERNL DWFPRMRAMS LVSSDSEGEQ NELRNLQEKL ESTMKLVTNL SGQLSELKDQ MTEQRKQK Q RIGLLGHPPH MNVNPQQPA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Details: 50 mM Tris-HCl buffer (pH 7.4), 150 mM NaCl, 1 mM DTT, 0.4% CHAPS, 2 mM EGTA, 1 mM EDTA, protease inhibitors |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Details: unavailable |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV |
| Details | 0.1 mg/ml |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Temperature | Min: 93.0 K / Max: 93.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 2-17 / Number real images: 9823 / Average exposure time: 0.2 sec. / Average electron dose: 1.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: GATAN 910 MULTI-SPECIMEN SINGLE TILT CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)