+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8987 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ApoCasX | |||||||||
 Map data Map data | ApoCasX | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Deltaproteobacteria (d-proteobacteria) Deltaproteobacteria (d-proteobacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 20.0 Å | |||||||||
 Authors Authors | Liu JJ / Orlova N | |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: CasX enzymes comprise a distinct family of RNA-guided genome editors. Authors: Jun-Jie Liu / Natalia Orlova / Benjamin L Oakes / Enbo Ma / Hannah B Spinner / Katherine L M Baney / Jonathan Chuck / Dan Tan / Gavin J Knott / Lucas B Harrington / Basem Al-Shayeb / ...Authors: Jun-Jie Liu / Natalia Orlova / Benjamin L Oakes / Enbo Ma / Hannah B Spinner / Katherine L M Baney / Jonathan Chuck / Dan Tan / Gavin J Knott / Lucas B Harrington / Basem Al-Shayeb / Alexander Wagner / Julian Brötzmann / Brett T Staahl / Kian L Taylor / John Desmarais / Eva Nogales / Jennifer A Doudna /   Abstract: The RNA-guided CRISPR-associated (Cas) proteins Cas9 and Cas12a provide adaptive immunity against invading nucleic acids, and function as powerful tools for genome editing in a wide range of ...The RNA-guided CRISPR-associated (Cas) proteins Cas9 and Cas12a provide adaptive immunity against invading nucleic acids, and function as powerful tools for genome editing in a wide range of organisms. Here we reveal the underlying mechanisms of a third, fundamentally distinct RNA-guided genome-editing platform named CRISPR-CasX, which uses unique structures for programmable double-stranded DNA binding and cleavage. Biochemical and in vivo data demonstrate that CasX is active for Escherichia coli and human genome modification. Eight cryo-electron microscopy structures of CasX in different states of assembly with its guide RNA and double-stranded DNA substrates reveal an extensive RNA scaffold and a domain required for DNA unwinding. These data demonstrate how CasX activity arose through convergent evolution to establish an enzyme family that is functionally separate from both Cas9 and Cas12a. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8987.map.gz emd_8987.map.gz | 3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8987-v30.xml emd-8987-v30.xml emd-8987.xml emd-8987.xml | 8.1 KB 8.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8987.png emd_8987.png | 31.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8987 http://ftp.pdbj.org/pub/emdb/structures/EMD-8987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8987 | HTTPS FTP |
-Related structure data
| Related structure data |  8980C  8988C  8989C  8990C  8991C  8994C  8996C  6ny1C  6ny2C  6ny3C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8987.map.gz / Format: CCP4 / Size: 3.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8987.map.gz / Format: CCP4 / Size: 3.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ApoCasX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.18 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : ApoCasX
| Entire | Name: ApoCasX |
|---|---|
| Components |
|
-Supramolecule #1: ApoCasX
| Supramolecule | Name: ApoCasX / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Deltaproteobacteria (d-proteobacteria) Deltaproteobacteria (d-proteobacteria) |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 46.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 80000 |
|---|---|
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller



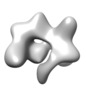






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















