+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-8606 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Bacteriophage P22 mature virion capsid protein | |||||||||
 マップデータ マップデータ | Bacteriophage P22 mature virion capsid protein, combined map | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | P22 Bacteriophage / VIRUS | |||||||||
| 機能・相同性 | Major capsid protein Gp5 / P22 coat protein - gene protein 5 / viral procapsid / viral procapsid maturation / T=7 icosahedral viral capsid / viral capsid / identical protein binding / Major capsid protein 機能・相同性情報 機能・相同性情報 | |||||||||
| 生物種 |  Salmonella phage P22 (ファージ) / Salmonella phage P22 (ファージ) /  Enterobacteria phage P22 (ファージ) Enterobacteria phage P22 (ファージ) | |||||||||
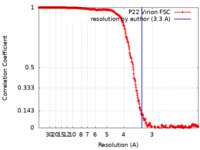
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.3 Å | |||||||||
 データ登録者 データ登録者 | Hryc CF / Chen D-H | |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2017 ジャーナル: Proc Natl Acad Sci U S A / 年: 2017タイトル: Accurate model annotation of a near-atomic resolution cryo-EM map. 著者: Corey F Hryc / Dong-Hua Chen / Pavel V Afonine / Joanita Jakana / Zhao Wang / Cameron Haase-Pettingell / Wen Jiang / Paul D Adams / Jonathan A King / Michael F Schmid / Wah Chiu /  要旨: Electron cryomicroscopy (cryo-EM) has been used to determine the atomic coordinates (models) from density maps of biological assemblies. These models can be assessed by their overall fit to the ...Electron cryomicroscopy (cryo-EM) has been used to determine the atomic coordinates (models) from density maps of biological assemblies. These models can be assessed by their overall fit to the experimental data and stereochemical information. However, these models do not annotate the actual density values of the atoms nor their positional uncertainty. Here, we introduce a computational procedure to derive an atomic model from a cryo-EM map with annotated metadata. The accuracy of such a model is validated by a faithful replication of the experimental cryo-EM map computed using the coordinates and associated metadata. The functional interpretation of any structural features in the model and its utilization for future studies can be made in the context of its measure of uncertainty. We applied this protocol to the 3.3-Å map of the mature P22 bacteriophage capsid, a large and complex macromolecular assembly. With this protocol, we identify and annotate previously undescribed molecular interactions between capsid subunits that are crucial to maintain stability in the absence of cementing proteins or cross-linking, as occur in other bacteriophages. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_8606.map.gz emd_8606.map.gz | 1.8 GB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-8606-v30.xml emd-8606-v30.xml emd-8606.xml emd-8606.xml | 26.3 KB 26.3 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_8606_fsc.xml emd_8606_fsc.xml | 30.3 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_8606.png emd_8606.png | 82.8 KB | ||
| Filedesc metadata |  emd-8606.cif.gz emd-8606.cif.gz | 9.2 KB | ||
| その他 |  emd_8606_half_map_1.map.gz emd_8606_half_map_1.map.gz emd_8606_half_map_2.map.gz emd_8606_half_map_2.map.gz | 1.8 GB 1.8 GB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8606 http://ftp.pdbj.org/pub/emdb/structures/EMD-8606 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8606 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8606 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  5uu5MC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | |
| 電子顕微鏡画像生データ |  EMPIAR-10083 (タイトル: Bacteriophage P22 mature virion capsid protein EMPIAR-10083 (タイトル: Bacteriophage P22 mature virion capsid proteinData size: 159.4 Data #1: Corrected images of P22 Mature Phage [picked particles - multiframe - processed]) |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_8606.map.gz / 形式: CCP4 / 大きさ: 2.4 GB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_8606.map.gz / 形式: CCP4 / 大きさ: 2.4 GB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Bacteriophage P22 mature virion capsid protein, combined map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
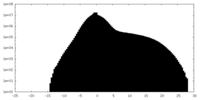
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
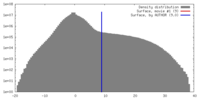
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-ハーフマップ: Bacteriophage P22 mature virion capsid protein, half map (set 0)
| ファイル | emd_8606_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Bacteriophage P22 mature virion capsid protein, half map (set 0) | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
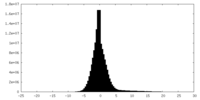
| 密度ヒストグラム |
-ハーフマップ: Bacteriophage P22 mature virion capsid protein, half map (set 1)
| ファイル | emd_8606_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Bacteriophage P22 mature virion capsid protein, half map (set 1) | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
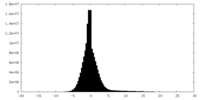
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Enterobacteria phage P22
| 全体 | 名称:  Enterobacteria phage P22 (ファージ) Enterobacteria phage P22 (ファージ) |
|---|---|
| 要素 |
|
-超分子 #1: Enterobacteria phage P22
| 超分子 | 名称: Enterobacteria phage P22 / タイプ: virus / ID: 1 / 親要素: 0 / 含まれる分子: all / NCBI-ID: 10754 / 生物種: Enterobacteria phage P22 / ウイルスタイプ: VIRION / ウイルス・単離状態: SPECIES / ウイルス・エンベロープ: No / ウイルス・中空状態: No |
|---|---|
| 分子量 | 理論値: 327.57294 MDa |
| ウイルス殻 | Shell ID: 1 / 名称: Capsid / 直径: 735.0 Å / T番号(三角分割数): 7 |
-分子 #1: Major capsid protein
| 分子 | 名称: Major capsid protein / タイプ: protein_or_peptide / ID: 1 / コピー数: 7 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Salmonella phage P22 (ファージ) Salmonella phage P22 (ファージ) |
| 分子量 | 理論値: 46.795613 KDa |
| 組換発現 | 生物種:  Salmonella enterica subsp. enterica serovar Typhimurium (サルモネラ菌) Salmonella enterica subsp. enterica serovar Typhimurium (サルモネラ菌) |
| 配列 | 文字列: MALNEGQIVT LAVDEIIETI SAITPMAQKA KKYTPPAASM QRSSNTIWMP VEQESPTQEG WDLTDKATGL LELNVAVNMG EPDNDFFQL RADDLRDETA YRRRIQSAAR KLANNVELKV ANMAAEMGSL VITSPDAIGT NTADAWNFVA DAEEIMFSRE L NRDMGTSY ...文字列: MALNEGQIVT LAVDEIIETI SAITPMAQKA KKYTPPAASM QRSSNTIWMP VEQESPTQEG WDLTDKATGL LELNVAVNMG EPDNDFFQL RADDLRDETA YRRRIQSAAR KLANNVELKV ANMAAEMGSL VITSPDAIGT NTADAWNFVA DAEEIMFSRE L NRDMGTSY FFNPQDYKKA GYDLTKRDIF GRIPEEAYRD GTIQRQVAGF DDVLRSPKLP VLTKSTATGI TVSGAQSFKP VA WQLDNDG NKVNVDNRFA TVTLSATTGM KRGDKISFAG VKFLGQMAKN VLAQDATFSV VRVVDGTHVE ITPKPVALDD VSL SPEQRA YANVNTSLAD AMAVNILNVK DARTNVFWAD DAIRIVSQPI PANHELFAGM KTTSFSIPDV GLNGIFATQG DIST LSGLC RIALWYGVNA TRPEAIGVGL PGQTA UniProtKB: Major capsid protein |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1 mg/mL |
|---|---|
| 緩衝液 | pH: 7.6 / 詳細: 50 mM Tris, pH 7.6, 1 mM MgCl2, 25 mM NaCl |
| グリッド | モデル: Quantifoil / 材質: COPPER / メッシュ: 400 / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 10 sec. |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 298 K / 装置: FEI VITROBOT MARK IV / 詳細: single blot, one second duration. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | JEOL 3200FSC |
|---|---|
| 温度 | 最低: 86.0 K / 最高: 87.0 K |
| 特殊光学系 | 色収差補正装置: none エネルギーフィルター - 名称: In-column Omega Filter エネルギーフィルター - エネルギー下限: 0 eV エネルギーフィルター - エネルギー上限: 20 eV |
| 詳細 | normal alignment |
| 撮影 | フィルム・検出器のモデル: DIRECT ELECTRON DE-20 (5k x 3k) 検出モード: INTEGRATING / デジタル化 - サイズ - 横: 5120 pixel / デジタル化 - サイズ - 縦: 3840 pixel / デジタル化 - 画像ごとのフレーム数: 1-6 / 撮影したグリッド数: 1 / 実像数: 2927 / 平均露光時間: 1.5 sec. / 平均電子線量: 37.5 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 100.0 µm / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 3.5 µm / 最小 デフォーカス(公称値): 1.5 µm / 倍率(公称値): 50000 |
| 試料ステージ | 試料ホルダーモデル: JEOL 3200FSC CRYOHOLDER / ホルダー冷却材: NITROGEN |
+ 画像解析
画像解析
-原子モデル構築 1
| 詳細 | In order to generate the atomic model, we fit our old model (PDB ID: 2XYZ) into subunit A, specifically the hexon capsid protein that sits at the two-fold axis with the penton subunit. To segment the capsid protein, a 30 Angstrom color zone in Chimera was used to separate the density. This ensured that any alteration in protein fold between the previous and current models would not be missed during the segmentation process. The segmented map was then imported into Coot and amino acid PRO25 was easily identified with a kink, similar to where it was previously located. This residue is located at the end of the N-arm and is predicted to be in a small helix which can be identified in the map. From there, baton building was done to the C-terminus and back to the N-terminus, completing the N-arm. Once complete, amino acids were mutated computationally one at a time and registration errors were adjusted based on visible density. All aromatic amino acids were visible and were used as anchor points for other amino acids that lacked strong positive density, such as negatively charged residues. The model was then optimized using the density as a constraint using Phenix.real_space_refine with default parameters, plus simulated annealing to encourage fit to density. Coot was then used to adjust various regions of the model that did not converge into the density such as a portion of the N-arm (weak density) and D-loop (containing several negatively charged amino acids with weak side-chain density). Real-space model optimization again followed, this time without simulated annealing. This model has two domains, the insertion domain and the P-domain / N-arm, that were interpreted differently in a previous model derived from a lower resolution map. We expected that improved resolution would alter the protein fold in the insertion domain. However, we did not anticipate any alterations in other regions of the model. When assessing the P-domain, we noted improved connectivity of the B-sheets in addition to a fourth strand which was previously modeled wrongly as the N-terminal helix due to poor resolvability. This fourth strand has never been seen in capsid proteins of the bacteriophage, and thus modeling it differently was understandable. When adjusting the threshold, a strand of density extending to the neighboring two-fold axis became visible from the PRO25 anchor point. The resulting model was placed into the density of the other six subunits in an asymmetric unit (ASU), and loops with large variations (the E-loop and a loop in the A-domain) were adjusted manually using Coot. Again, real-space model optimization of the complex followed using default parameters. The refined ASU was then surrounded by neighboring asymmetric units, ensuring that clashes would be avoided and interactions optimized. Coot was then used to manually adjust any rotamers or regions with poor geometry. Moreover, Phenix.molprobity was run to generate a Coot import file, allowing for the removal of extreme clashes and Ramachandran outliers. Finally, a second round of real-space model optimization was completed with simulated annealing and morphing applied. This allowed for greater freedom of model movement. A final MolProbity check was completed to assess stereochemistry. To assess fit to density, cross-correlation was computed during phenix.real_space_refine, and an EMRinger was computed. Model to Calculated Map: To generate a weighted map from the model that would represent the experimental density map, both occupancies and ADPs had to be refined against the experimental map. ADPs were first set to 50 (Angstrom)^2 for all amino acids in our ASU - with surrounding subunits - and refined with phenix.real_space_refine (run=adp). Two iterations were performed to insure convergence. Occupancies were then changed to -0.5 for all carboxyl oxygens in the refined complex. This negative value was needed in order to produce negative density in the map calculated from the model. These occupancies do not refer to the absence of atoms, but rather are used as weighting values due to the lack of a proper form factor. Occupancies were then refined with phenix.refine in reciprocal space, which resulted in some occupancies reverting to positivevalues. An additional iteration of ADP and occupancy refinement then followed. With atom positions, ADPs, and occupancies all refined, a map was calculated from the model at 3.3 Angstrom resolution using Phenix.fmodel and converted to a CCP4 format map using phenix.mtz2map. Model Validation / Uncertainty: The generation of two independent models optimized for the two half maps is a validation practice which assures that agreement is consistent with the claimed 3.3 Angstrom resolution as reflected by the FSC=0.5 numerical value suggested previously. Moreover, assessment of independent models provides an understanding of the level of uncertainty within the map. Both half data sets (~3.4 Angstrom resolution) were modeled independently. Model variation to assess uncertainty was computed in Chimera, and the FSC was computed with EMAN2. |
|---|---|
| 精密化 | 空間: REAL / プロトコル: AB INITIO MODEL / 温度因子: 0.836 |
| 得られたモデル |  PDB-5uu5: |
 ムービー
ムービー コントローラー
コントローラー
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)