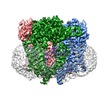
登録情報 データベース : EMDB / ID : EMD-8354タイトル Cryo-EM structure of Polycystic Kidney Disease protein 2 (PKD2), residues 198-703 B-factor sharpened, masked map generated from relion auto-refinement and postprocessing 複合体 : hPKD:198-703タンパク質・ペプチド : hPKD:198-703, Polycystin-2リガンド : 2-acetamido-2-deoxy-beta-D-glucopyranose / / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
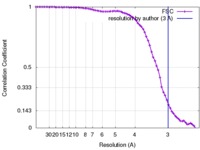
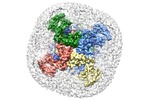
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Homo sapiens (ヒト)手法 / / 解像度 : 3.0 Å Shen PS / Yang X / DeCaen PG / Liu X / Bulkley D / Clapham DE / Cao E ジャーナル : Cell / 年 : 2016タイトル : The Structure of the Polycystic Kidney Disease Channel PKD2 in Lipid Nanodiscs.著者 : Peter S Shen / Xiaoyong Yang / Paul G DeCaen / Xiaowen Liu / David Bulkley / David E Clapham / Erhu Cao / 要旨 : The Polycystic Kidney Disease 2 (Pkd2) gene is mutated in autosomal dominant polycystic kidney disease (ADPKD), one of the most common human monogenic disorders. Here, we present the cryo-EM ... The Polycystic Kidney Disease 2 (Pkd2) gene is mutated in autosomal dominant polycystic kidney disease (ADPKD), one of the most common human monogenic disorders. Here, we present the cryo-EM structure of PKD2 in lipid bilayers at 3.0 Å resolution, which establishes PKD2 as a homotetrameric ion channel and provides insight into potential mechanisms for its activation. The PKD2 voltage-sensor domain retains two of four gating charges commonly found in those of voltage-gated ion channels. The PKD2 ion permeation pathway is constricted at the selectivity filter and near the cytoplasmic end of S6, suggesting that two gates regulate ion conduction. The extracellular domain of PKD2, a hotspot for ADPKD pathogenic mutations, contributes to channel assembly and strategically interacts with the transmembrane core, likely serving as a physical substrate for extracellular stimuli to allosterically gate the channel. Finally, our structure establishes the molecular basis for the majority of pathogenic mutations in Pkd2-related ADPKD. 履歴 登録 2016年9月27日 - ヘッダ(付随情報) 公開 2016年10月26日 - マップ公開 2016年11月2日 - 更新 2024年11月13日 - 現状 2024年11月13日 処理サイト : RCSB / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 引用
引用 ジャーナル: Cell / 年: 2016
ジャーナル: Cell / 年: 2016
 構造の表示
構造の表示 ムービービューア
ムービービューア SurfView
SurfView Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク emd_8354.map.gz
emd_8354.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-8354-v30.xml
emd-8354-v30.xml emd-8354.xml
emd-8354.xml EMDBヘッダ
EMDBヘッダ emd_8354_fsc.xml
emd_8354_fsc.xml FSCデータファイル
FSCデータファイル emd_8354_1.png
emd_8354_1.png emd_8354_2.png
emd_8354_2.png emd_8354_msk_1.map
emd_8354_msk_1.map マスクマップ
マスクマップ emd-8354.cif.gz
emd-8354.cif.gz emd_8354_half_map_1.map.gz
emd_8354_half_map_1.map.gz emd_8354_half_map_2.map.gz
emd_8354_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-8354
http://ftp.pdbj.org/pub/emdb/structures/EMD-8354 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8354
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8354 emd_8354_validation.pdf.gz
emd_8354_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_8354_full_validation.pdf.gz
emd_8354_full_validation.pdf.gz emd_8354_validation.xml.gz
emd_8354_validation.xml.gz emd_8354_validation.cif.gz
emd_8354_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8354
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8354 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8354
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8354 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_8354.map.gz / 形式: CCP4 / 大きさ: 27 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_8354.map.gz / 形式: CCP4 / 大きさ: 27 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) emd_8354_msk_1.map
emd_8354_msk_1.map 試料の構成要素
試料の構成要素 Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト)
 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 ムービー
ムービー コントローラー
コントローラー















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)