+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Capsid of Vibrio cholerae phage mature VP1 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | phage / virus / vibrio cholera phage / VIRAL PROTEIN | ||||||||||||
| Biological species |   | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | ||||||||||||
 Authors Authors | Liu HR / Pang H | ||||||||||||
| Funding support |  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Three-dimensional structures of Vibrio cholerae typing podophage VP1 in two states. Authors: Hao Pang / Fenxia Fan / Jing Zheng / Hao Xiao / Zhixue Tan / Jingdong Song / Biao Kan / Hongrong Liu /  Abstract: Lytic podophages (VP1-VP5) play crucial roles in subtyping Vibrio cholerae O1 biotype El Tor. However, until now no structures of these phages have been available, which hindered our understanding of ...Lytic podophages (VP1-VP5) play crucial roles in subtyping Vibrio cholerae O1 biotype El Tor. However, until now no structures of these phages have been available, which hindered our understanding of the molecular mechanisms of infection and DNA release. Here, we determined the cryoelectron microscopy (cryo-EM) structures of mature and DNA-ejected VP1 structures at near-atomic and subnanometer resolutions, respectively. The VP1 head is composed of 415 copies of the major capsid protein gp7 and 11 turret-shaped spikes. The VP1 tail consists of an adapter, a nozzle, a slender ring, and a tail needle, and is flanked by three extended fibers I and six trimeric fibers II. Conformational changes of fiber II in DNA-ejected VP1 may cause the release of the tail needle and core proteins, forming an elongated tail channel. Our structures provide insights into the molecular mechanisms of infection and DNA release for podophages with a tail needle. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_60702.map.gz emd_60702.map.gz | 267.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-60702-v30.xml emd-60702-v30.xml emd-60702.xml emd-60702.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_60702.png emd_60702.png | 127.9 KB | ||
| Filedesc metadata |  emd-60702.cif.gz emd-60702.cif.gz | 5.9 KB | ||
| Others |  emd_60702_half_map_1.map.gz emd_60702_half_map_1.map.gz emd_60702_half_map_2.map.gz emd_60702_half_map_2.map.gz | 268.9 MB 268.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-60702 http://ftp.pdbj.org/pub/emdb/structures/EMD-60702 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60702 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60702 | HTTPS FTP |
-Related structure data
| Related structure data |  9in6MC  8zkkC  8zkmC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_60702.map.gz / Format: CCP4 / Size: 290.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_60702.map.gz / Format: CCP4 / Size: 290.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
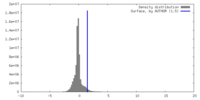
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_60702_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
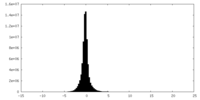
| Density Histograms |
-Half map: #2
| File | emd_60702_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
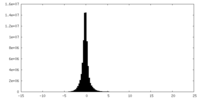
| Density Histograms |
- Sample components
Sample components
-Entire : Vibrio cholerae phage
| Entire | Name:  |
|---|---|
| Components |
|
-Supramolecule #1: Vibrio cholerae phage
| Supramolecule | Name: Vibrio cholerae phage / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 666 / Sci species name: Vibrio cholerae phage / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: major capsid of VP1
| Macromolecule | Name: major capsid of VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 43.886555 KDa |
| Sequence | String: MATIAGLRGT GDWGNQERPT DFRETILWME PNGQAPLQAL MSKMSSQPTT DPEFSWWEEK LTHNRLEVKT EAAAGVTTLA VDTDQAWAC VKGDILMVES VGGLWANEIL KVVEDPTAGN ALKVARGFAG TTAAVIPAGT FIIAIGTSFA EGSLAPKSAT R NPVKLNNF ...String: MATIAGLRGT GDWGNQERPT DFRETILWME PNGQAPLQAL MSKMSSQPTT DPEFSWWEEK LTHNRLEVKT EAAAGVTTLA VDTDQAWAC VKGDILMVES VGGLWANEIL KVVEDPTAGN ALKVARGFAG TTAAVIPAGT FIIAIGTSFA EGSLAPKSAT R NPVKLNNF CQIFKKSYEI TKTADATKAR TGSALANDKK RRMFDYYRDV EMAFIYGRKS ETVGENGKPE RTTGGLLNFI TT NRTQFGT GAGKTELTED SLIDFFANVF NYDGQGAGNQ RIAFVGNTAL TKINKLARNS PSTRINFDKQ VTQVYGMNFT RWV LPQGEI FFKTHPLFNV HPELSKAMMV LNPKGIKERV LRATKPENDI QQVGQDSIKG QWIGEFGLEV NHEETMAFAG GIA |
-Macromolecule #2: SHP of VP1
| Macromolecule | Name: SHP of VP1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 11.801501 KDa |
| Sequence | String: MLVHYGKLSD MPTVVDVNTT MGTDVPEDLL EIYVGCYAAD GKTPAAGTGV LTFHGSWNGV HKRLIGTVDL AAAGEVIAYN PPLMFGGCK KLFVSYTGVG TQIVDVYVHR GE |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOCONTINUUM (6k x 4k) / Average electron dose: 32.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)