+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | scFv antibody CL33 bound to R-phycoerythrin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Phycoerythrin / antibody / scFv / immunity / IMMUNE SYSTEM | |||||||||
| Biological species |  Neopyropia tenera (asakusa nori) / Neopyropia tenera (asakusa nori) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.1 Å | |||||||||
 Authors Authors | Rashleigh L / Gully BS | |||||||||
| Funding support |  Australia, 1 items Australia, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2025 Journal: Structure / Year: 2025Title: Antibody-like recognition of a γδ T cell receptor toward a foreign antigen. Authors: Liam Rashleigh / Hariprasad Venugopal / Michael T Rice / Sachith D Gunasinghe / Chhon Ling Sok / Nicholas A Gherardin / Catarina F Almeida / Ildiko Van Rhijn / D Branch Moody / Dale I ...Authors: Liam Rashleigh / Hariprasad Venugopal / Michael T Rice / Sachith D Gunasinghe / Chhon Ling Sok / Nicholas A Gherardin / Catarina F Almeida / Ildiko Van Rhijn / D Branch Moody / Dale I Godfrey / Jamie Rossjohn / Benjamin S Gully /    Abstract: The antigen recognition principles of B cells and αβ T cells have been well described compared to those of the γδ T cell. By way of their specificity conferring receptor (γδTCR), γδ T cells ...The antigen recognition principles of B cells and αβ T cells have been well described compared to those of the γδ T cell. By way of their specificity conferring receptor (γδTCR), γδ T cells can directly bind proteinaceous antigens. A known γδ T cell and B cell model antigen is phycoerythrin (PE), a light harvesting protein from rhodophytes and cyanobacteria. Here we probed human γδTCR reactivity to PE, in which a Vδ1Vγ5 TCR bound directly to induce proximal signaling and cellular activation. We determined the cryoelectron microscopy (cryo-EM) structure of the γδTCR-phycoerythrin immune complex. We then determined the cryo-EM structures of an antibody fragment and an αβTCR bound to PE. This revealed convergent use of apical aromatic residues to mediate contacts with a common PE epitope. Comparative analyses of the γδTCR revealed multiple antibody-like characteristics, including an enrichment of apical aromatic residues. Our findings reveal further distinct facets of antigen recognition by the γδTCR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_48248.map.gz emd_48248.map.gz | 89.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-48248-v30.xml emd-48248-v30.xml emd-48248.xml emd-48248.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_48248_fsc.xml emd_48248_fsc.xml | 11.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_48248.png emd_48248.png | 102.3 KB | ||
| Filedesc metadata |  emd-48248.cif.gz emd-48248.cif.gz | 6.2 KB | ||
| Others |  emd_48248_additional_1.map.gz emd_48248_additional_1.map.gz emd_48248_half_map_1.map.gz emd_48248_half_map_1.map.gz emd_48248_half_map_2.map.gz emd_48248_half_map_2.map.gz | 168 MB 165.2 MB 165.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-48248 http://ftp.pdbj.org/pub/emdb/structures/EMD-48248 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-48248 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-48248 | HTTPS FTP |
-Related structure data
| Related structure data |  9mgbMC  9mkoC  9o60C  9o61C  9o62C M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_48248.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_48248.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.94 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_48248_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_48248_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_48248_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : R-phycoerythrin bound by CL33 scFv
| Entire | Name: R-phycoerythrin bound by CL33 scFv |
|---|---|
| Components |
|
-Supramolecule #1: R-phycoerythrin bound by CL33 scFv
| Supramolecule | Name: R-phycoerythrin bound by CL33 scFv / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: R-phycoerythrin
| Supramolecule | Name: R-phycoerythrin / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Neopyropia tenera (asakusa nori) Neopyropia tenera (asakusa nori) |
-Supramolecule #3: CL33 scFV
| Supramolecule | Name: CL33 scFV / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: R-phycoerythrin alpha chain
| Macromolecule | Name: R-phycoerythrin alpha chain / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neopyropia tenera (asakusa nori) Neopyropia tenera (asakusa nori) |
| Molecular weight | Theoretical: 17.707906 KDa |
| Sequence | String: MKSVITTTIS AADAAGRFPS SSDLESVQGN IQRAASRLEA AEKLAGNHEA VVKEAGDACF AKYPYLKNPG EAGDSQEKIN KCYRDIDHY MRLINYSLVV GGTGPLDEWG IAGAREVYRA LNLPGSSYIA AFVFTRDRLC VPRDMSAQAA VEFSGALDYV I NSLC |
-Macromolecule #2: R-phycoerythrin beta chain
| Macromolecule | Name: R-phycoerythrin beta chain / type: protein_or_peptide / ID: 2 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neopyropia tenera (asakusa nori) Neopyropia tenera (asakusa nori) |
| Molecular weight | Theoretical: 18.398875 KDa |
| Sequence | String: MLDAFSRVVV NSDSKAAYVS GSDLQALKTF IADGNKRLDA VNSIVSNASC IVSDAVSGMI CENPGLIAPG GNCYTNRRMA ACLRDGEII LRYTSYALLA GDSSVLEDRC LNGLKETYIA LGVPTNSTVR AVSIMKSSAV AFISNTASQR KMATADGDCS A LSSEVASY CDKVSAAI |
-Macromolecule #3: CL33 scFv
| Macromolecule | Name: CL33 scFv / type: protein_or_peptide / ID: 3 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 27.609285 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVHSEVQLQQ SGAELARPGA SVKLSCKASG YTFTSYGISW VKQRTGQGLE WIGEIYPRSG NTYYNEKFKG KATLTADKSS STAYMELRS LTSEDSAVYF CARQGYYANS QFTYWGQGTL VTVSAAAGGG GSGGGGSGGG GSVHSDIQMT QTTSSLSASL G DRVTISCS ...String: MVHSEVQLQQ SGAELARPGA SVKLSCKASG YTFTSYGISW VKQRTGQGLE WIGEIYPRSG NTYYNEKFKG KATLTADKSS STAYMELRS LTSEDSAVYF CARQGYYANS QFTYWGQGTL VTVSAAAGGG GSGGGGSGGG GSVHSDIQMT QTTSSLSASL G DRVTISCS ASQGISNYLN WYQQKPDGTV KLLIYYTSSL HSGVPSRFSG SGSGTDYSLT ISNLEPEDIA TYYCQQYSKL PW TFGGGTN LEIKHHHHHH |
-Macromolecule #4: PHYCOERYTHROBILIN
| Macromolecule | Name: PHYCOERYTHROBILIN / type: ligand / ID: 4 / Number of copies: 24 / Formula: PEB |
|---|---|
| Molecular weight | Theoretical: 588.694 Da |
| Chemical component information |  ChemComp-PEB: |
-Macromolecule #5: PHYCOUROBILIN
| Macromolecule | Name: PHYCOUROBILIN / type: ligand / ID: 5 / Number of copies: 6 / Formula: PUB |
|---|---|
| Molecular weight | Theoretical: 590.71 Da |
| Chemical component information | 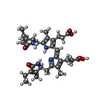 ChemComp-CYB: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)













































