+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4716 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | BACTERIOPHAGE SPP1 MATURE CAPSID PROTEIN | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bacteriophage / Capsid protein / image processing / VIRUS | |||||||||
| Function / homology | Major capsid protein 13-like / Major capsid protein 13-like / T=7 icosahedral viral capsid / viral capsid / Major capsid protein Function and homology information Function and homology information | |||||||||
| Biological species |  Bacillus phage SPP1 (virus) Bacillus phage SPP1 (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.0 Å | |||||||||
 Authors Authors | Ignatiou A / El Sadek Fadel M | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Structural transitions during the scaffolding-driven assembly of a viral capsid. Authors: Athanasios Ignatiou / Sandrine Brasilès / Mehdi El Sadek Fadel / Jörg Bürger / Thorsten Mielke / Maya Topf / Paulo Tavares / Elena V Orlova /    Abstract: Assembly of tailed bacteriophages and herpesviruses starts with formation of procapsids (virion precursors without DNA). Scaffolding proteins (SP) drive assembly by chaperoning the major capsid ...Assembly of tailed bacteriophages and herpesviruses starts with formation of procapsids (virion precursors without DNA). Scaffolding proteins (SP) drive assembly by chaperoning the major capsid protein (MCP) to build an icosahedral lattice. Here we report near-atomic resolution cryo-EM structures of the bacteriophage SPP1 procapsid, the intermediate expanded procapsid with partially released SPs, and the mature capsid with DNA. In the intermediate state, SPs are bound only to MCP pentons and to adjacent subunits from hexons. SP departure results in the expanded state associated with unfolding of the MCP N-terminus and straightening of E-loops. The newly formed extensive inter-capsomere bonding appears to compensate for release of SPs that clasp MCP capsomeres together. Subsequent DNA packaging instigates bending of MCP A domain loops outwards, closing the hexons central opening and creating the capsid auxiliary protein binding interface. These findings provide a molecular basis for the sequential structural rearrangements during viral capsid maturation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4716.map.gz emd_4716.map.gz | 115.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4716-v30.xml emd-4716-v30.xml emd-4716.xml emd-4716.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4716.png emd_4716.png | 446.8 KB | ||
| Filedesc metadata |  emd-4716.cif.gz emd-4716.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4716 http://ftp.pdbj.org/pub/emdb/structures/EMD-4716 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4716 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4716 | HTTPS FTP |
-Related structure data
| Related structure data |  6r3aMC  4717C  6r3bC  6rtlC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4716.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4716.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
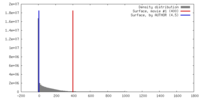
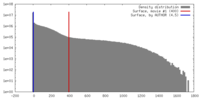
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Bacillus phage SPP1
| Entire | Name:  Bacillus phage SPP1 (virus) Bacillus phage SPP1 (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Bacillus phage SPP1
| Supramolecule | Name: Bacillus phage SPP1 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: Mature assembly, icosahedral symmetry has been applied NCBI-ID: 10724 / Sci species name: Bacillus phage SPP1 / Sci species strain: SPP1sus70 / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Bactria (plant) / Strain: Bacillus Bactria (plant) / Strain: Bacillus |
| Molecular weight | Theoretical: 30 MDa |
| Virus shell | Shell ID: 1 / Name: capsid / Diameter: 610.0 Å / T number (triangulation number): 7 |
-Macromolecule #1: Major capsid protein
| Macromolecule | Name: Major capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bacillus phage SPP1 (virus) Bacillus phage SPP1 (virus) |
| Molecular weight | Theoretical: 35.258426 KDa |
| Sequence | String: AYTKISDVIV PELFNPYVIN TTTQLSAFFQ SGIAATDDEL NALAKKAGGG STLNMPYWND LDGDSQVLND TDDLVPQKIN AGQDKAVLI LRGNAWSSHD LAATLSGSDP MQAIGSRVAA YWAREMQKIV FAELAGVFSN DDMKDNKLDI SGTADGIYSA E TFVDASYK ...String: AYTKISDVIV PELFNPYVIN TTTQLSAFFQ SGIAATDDEL NALAKKAGGG STLNMPYWND LDGDSQVLND TDDLVPQKIN AGQDKAVLI LRGNAWSSHD LAATLSGSDP MQAIGSRVAA YWAREMQKIV FAELAGVFSN DDMKDNKLDI SGTADGIYSA E TFVDASYK LGDHESLLTA IGMHSATMAS AVKQDLIEFV KDSQSGIRFP TYMNKRVIVD DSMPVETLED GTKVFTSYLF GA GALGYAE GQPEVPTETA RNALGSQDIL INRKHFVLHP RGVKFTENAM AGTTPTDEEL ANGANWQRVY DPKKIRIVQF KHR LQA UniProtKB: Major capsid protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R3/3 / Material: COPPER / Mesh: 400 / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK II |
| Details | Mature virions of the SPP1 bacteriophage |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Temperature | Min: 50.0 K / Max: 60.0 K |
| Specialist optics | Chromatic aberration corrector: none |
| Details | Sample preparation was screened in advance |
| Image recording | Film or detector model: GATAN K2 BASE (4k x 4k) / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 1 / Number real images: 7000 / Average exposure time: 5.0 sec. / Average electron dose: 25.0 e/Å2 Details: Images were collected in movie mode, 25 images during 5 s |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 3.5 µm / Calibrated defocus min: 1.0 µm / Calibrated magnification: 31000 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.3 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 31000 |
| Sample stage | Specimen holder model: GATAN 910 MULTI-SPECIMEN SINGLE TILT CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-6r3a: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)