+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4414 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | beta-galactosidase (integrating mode) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
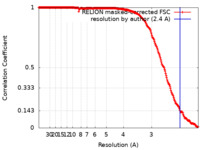
| Method | single particle reconstruction / cryo EM / Resolution: 2.4 Å | |||||||||
 Authors Authors | Song B / Flegler V | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Ultramicroscopy / Year: 2019 Journal: Ultramicroscopy / Year: 2019Title: Capabilities of the Falcon III detector for single-particle structure determination. Authors: Boyuan Song / Julian Lenhart / Vanessa Judith Flegler / Cihan Makbul / Tim Rasmussen / Bettina Böttcher /  Abstract: Direct electron detectors are an essential asset for the resolution revolution in electron cryo microscopy of biological objects. The direct detectors provide two modes of data acquisition; the ...Direct electron detectors are an essential asset for the resolution revolution in electron cryo microscopy of biological objects. The direct detectors provide two modes of data acquisition; the counting mode in which single electrons are counted, and the integrating mode in which the signal that arises from the incident electrons is integrated. While counting mode leads to far higher detective quantum efficiency at all spatial frequencies, the integrating mode enables faster data acquisition at higher exposure rates. For optimal throughput at best possible resolution it is important to understand when the better performance in counting mode becomes essential for solving a structure and when the lower detective quantum efficiency in integrating mode can be compensated by increasing the number of particles in the data set. Here, we provide a case study of the Falcon III camera, which has counting mode capability at exposure rates of <0.9 e/Px² and integrating mode capability at exposure rates above 10 e/Px². We found that counting mode gives better resolution for medium sized complexes such as the β-galactosidase (465 kDa) (2.2 Å, 97% of Nyquist vs. 2.4 Å, 89% of Nyquist) with data sets of similar size. However, for larger particles such as Hepatitis B virus capsid like particles (4.8 MDa) we did not find any resolution gain in counting mode. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4414.map.gz emd_4414.map.gz | 767.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4414-v30.xml emd-4414-v30.xml emd-4414.xml emd-4414.xml | 19.2 KB 19.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4414_fsc.xml emd_4414_fsc.xml | 21 KB | Display |  FSC data file FSC data file |
| Images |  emd_4414.png emd_4414.png | 102.7 KB | ||
| Others |  emd_4414_half_map_1.map.gz emd_4414_half_map_1.map.gz emd_4414_half_map_2.map.gz emd_4414_half_map_2.map.gz | 666.9 MB 666.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4414 http://ftp.pdbj.org/pub/emdb/structures/EMD-4414 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4414 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4414 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4414.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4414.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0635 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
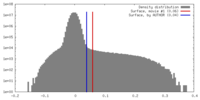
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #2
| File | emd_4414_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
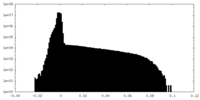
| Projections & Slices |
| ||||||||||||
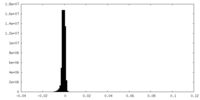
| Density Histograms |
-Half map: #1
| File | emd_4414_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
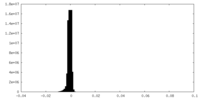
| Density Histograms |
- Sample components
Sample components
-Entire : beta-galactosidase
| Entire | Name: beta-galactosidase |
|---|---|
| Components |
|
-Supramolecule #1: beta-galactosidase
| Supramolecule | Name: beta-galactosidase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: sample was purchased from Sigma G5635 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 465 KDa |
-Macromolecule #1: beta-galactosidase
| Macromolecule | Name: beta-galactosidase / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: beta-galactosidase |
|---|---|
| Sequence | String: MTMITDSLAV VLQRRDWENP GVTQLNRLAA HPPFASWRNS EEARTDRPSQ QLRSLNGEWR FAWFPAPEA VPESWLECDL PEADTVVVPS NWQMHGYDAP IYTNVTYPIT VNPPFVPTEN P TGCYSLTF NVDESWLQEG QTRIIFDGVN SAFHLWCNGR WVGYGQDSRL ...String: MTMITDSLAV VLQRRDWENP GVTQLNRLAA HPPFASWRNS EEARTDRPSQ QLRSLNGEWR FAWFPAPEA VPESWLECDL PEADTVVVPS NWQMHGYDAP IYTNVTYPIT VNPPFVPTEN P TGCYSLTF NVDESWLQEG QTRIIFDGVN SAFHLWCNGR WVGYGQDSRL PSEFDLSAFL RA GENRLAV MVLRWSDGSY LEDQDMWRMS GIFRDVSLLH KPTTQISDFH VATRFNDDFS RAV LEAEVQ MCGELRDYLR VTVSLWQGET QVASGTAPFG GEIIDERGGY ADRVTLRLNV ENPK LWSAE IPNLYRAVVE LHTADGTLIE AEACDVGFRE VRIENGLLLL NGKPLLIRGV NRHEH HPLH GQVMDEQTMV QDILLMKQNN FNAVRCSHYP NHPLWYTLCD RYGLYVVDEA NIETHG MVP MNRLTDDPRW LPAMSERVTR MVQRDRNHPS VIIWSLGNES GHGANHDALY RWIKSVD PS RPVQYEGGGA DTTATDIICP MYARVDEDQP FPAVPKWSIK KWLSLPGETR PLILCEYA H AMGNSLGGFA KYWQAFRQYP RLQGGFVWDW VDQSLIKYDE NGNPWSAYGG DFGDTPNDR QFCMNGLVFA DRTPHPALTE AKHQQQFFQF RLSGQTIEVT SEYLFRHSDN ELLHWMVALD GKPLASGEV PLDVAPQGKQ LIELPELPQP ESAGQLWLTV RVVQPNATAW SEAGHISAWQ Q WRLAENLS VTLPAASHAI PHLTTSEMDF CIELGNKRWQ FNRQSGFLSQ MWIGDKKQLL TP LRDQFTR APLDNDIGVS EATRIDPNAW VERWKAAGHY QAEAALLQCT ADTLADAVLI TTA HAWQHQ GKTLFISRKT YRIDGSGQMA ITVDVEVASD TPHPARIGLN CQLAQVAERV NWLG LGPQE NYPDRLTAAC FDRWDLPLSD MYTPYVFPSE NGLRCGTREL NYGPHQWRGD FQFNI SRYS QQQLMETSHR HLLHAEEGTW LNIDGFHMGI GGDDSWSPSV SAEFQLSAGR YHYQLV WCQ K |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.6 mg/mL |
|---|---|
| Buffer | pH: 7.9 Details: The lyophilized protein powder was reconstituted into a 5 mg/ml solution with 50 mM Tris-HCl (pH 7.9), 50 mM NaCl, 2 mM MgCl2 and 2mM Mercaptoethanol. 300 ul of this solution were loaded ...Details: The lyophilized protein powder was reconstituted into a 5 mg/ml solution with 50 mM Tris-HCl (pH 7.9), 50 mM NaCl, 2 mM MgCl2 and 2mM Mercaptoethanol. 300 ul of this solution were loaded onto a Superdex 200 10/300 GL (GE LifeSciences) size-exclusion chromatography column and eluted with 50 mM Tris-HCl (pH 7.9), 50 mM NaCl, 2 mM MgCl2 and 2 mM TCEP. Peak fractions corresponding to the tetrameric complex were pooled and concentrated to 2.6 mg/ml with a 50 kDa MWCO spin concentrator (Amicon, Millipore Co.). The sample were checked by SDS-PAGE with silver staining (Pierce silver staining kit, ThermoFischer Scientific). |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: Quantifoil 400 mesh copper grids R 1.2/1.3 were used (Quantifoil Micro Tools GmbH, Jena, Germany). Grids were glow discharged in air at a pressure of 2.2x10-2 Torr for 2 min at medium power ...Details: Quantifoil 400 mesh copper grids R 1.2/1.3 were used (Quantifoil Micro Tools GmbH, Jena, Germany). Grids were glow discharged in air at a pressure of 2.2x10-2 Torr for 2 min at medium power with a Harrick Plasma cleaner (PDC-002). Subsequently, 3-4 ul of the sample was pipetted onto the glow discharged grids and plunge frozen in liquid Ethane with a Vitrobot IV (FEI). The sample chamber of the Vitrobot was maintained at 4C and 100% humidity. Wait time between sample application and blotting was 45 s, the drain time 0 s, the blot time 3 s and the blot force 0.. |
| Details | beta-Galactosidase from E. coli was purchased from SIGMA-ALDRICH (G5635). |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 79.0 K / Max: 80.0 K |
| Details | data has been recorded in integrating mode 25 frames per movie |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 1389 / Average exposure time: 4.3 sec. / Average electron dose: 52.0 e/Å2 Details: images were collected in movie mode (25 frames in total) |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 2.77 µm / Calibrated defocus min: 1.12 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: AB INITIO MODEL / Overall B value: 34 |
|---|
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)