+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of PKA phosphorylated human RyR2-R420W in the primed state | |||||||||
 マップデータ マップデータ | Composite map of the Structure of PKA phosphorylated human RyR2-R420W in the primed state | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | calcium channel / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報junctional sarcoplasmic reticulum membrane / sarcoplasmic reticulum calcium ion transport / establishment of protein localization to endoplasmic reticulum / type B pancreatic cell apoptotic process / Purkinje myocyte to ventricular cardiac muscle cell signaling / calcium-induced calcium release activity / regulation of atrial cardiac muscle cell action potential / left ventricular cardiac muscle tissue morphogenesis / suramin binding / regulation of AV node cell action potential ...junctional sarcoplasmic reticulum membrane / sarcoplasmic reticulum calcium ion transport / establishment of protein localization to endoplasmic reticulum / type B pancreatic cell apoptotic process / Purkinje myocyte to ventricular cardiac muscle cell signaling / calcium-induced calcium release activity / regulation of atrial cardiac muscle cell action potential / left ventricular cardiac muscle tissue morphogenesis / suramin binding / regulation of AV node cell action potential / regulation of SA node cell action potential / cell communication by electrical coupling involved in cardiac conduction / regulation of ventricular cardiac muscle cell action potential / ventricular cardiac muscle cell action potential / positive regulation of sequestering of calcium ion / negative regulation of calcium-mediated signaling / embryonic heart tube morphogenesis / cardiac muscle hypertrophy / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / insulin secretion involved in cellular response to glucose stimulus / calcium ion transport into cytosol / ryanodine-sensitive calcium-release channel activity / response to caffeine / regulation of cardiac muscle contraction by calcium ion signaling / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / response to redox state / 'de novo' protein folding / cellular response to caffeine / negative regulation of heart rate / FK506 binding / response to muscle activity / protein kinase A regulatory subunit binding / protein kinase A catalytic subunit binding / positive regulation of the force of heart contraction / smooth endoplasmic reticulum / intracellularly gated calcium channel activity / smooth muscle contraction / detection of calcium ion / regulation of cardiac muscle contraction / regulation of cytosolic calcium ion concentration / T cell proliferation / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / positive regulation of heart rate / calcium channel inhibitor activity / Ion homeostasis / cardiac muscle contraction / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / response to muscle stretch / release of sequestered calcium ion into cytosol / cellular response to epinephrine stimulus / calcium channel complex / sarcoplasmic reticulum membrane / regulation of heart rate / sarcoplasmic reticulum / protein maturation / peptidylprolyl isomerase / calcium channel regulator activity / peptidyl-prolyl cis-trans isomerase activity / establishment of localization in cell / Stimuli-sensing channels / calcium-mediated signaling / sarcolemma / calcium channel activity / Z disc / intracellular calcium ion homeostasis / calcium ion transport / positive regulation of cytosolic calcium ion concentration / protein refolding / transmembrane transporter binding / calmodulin binding / response to hypoxia / signaling receptor binding / calcium ion binding / enzyme binding / protein-containing complex / identical protein binding / membrane / plasma membrane / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.13 Å | |||||||||
 データ登録者 データ登録者 | Miotto MC / Marks AR | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2024 ジャーナル: Nat Commun / 年: 2024タイトル: Structural basis for ryanodine receptor type 2 leak in heart failure and arrhythmogenic disorders. 著者: Marco C Miotto / Steven Reiken / Anetta Wronska / Qi Yuan / Haikel Dridi / Yang Liu / Gunnar Weninger / Carl Tchagou / Andrew R Marks /  要旨: Heart failure, the leading cause of mortality and morbidity in the developed world, is characterized by cardiac ryanodine receptor 2 channels that are hyperphosphorylated, oxidized, and depleted of ...Heart failure, the leading cause of mortality and morbidity in the developed world, is characterized by cardiac ryanodine receptor 2 channels that are hyperphosphorylated, oxidized, and depleted of the stabilizing subunit calstabin-2. This results in a diastolic sarcoplasmic reticulum Ca leak that impairs cardiac contractility and triggers arrhythmias. Genetic mutations in ryanodine receptor 2 can also cause Ca leak, leading to arrhythmias and sudden cardiac death. Here, we solved the cryogenic electron microscopy structures of ryanodine receptor 2 variants linked either to heart failure or inherited sudden cardiac death. All are in the primed state, part way between closed and open. Binding of Rycal drugs to ryanodine receptor 2 channels reverts the primed state back towards the closed state, decreasing Ca leak, improving cardiac function, and preventing arrhythmias. We propose a structural-physiological mechanism whereby the ryanodine receptor 2 channel primed state underlies the arrhythmias in heart failure and arrhythmogenic disorders. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_42762.map.gz emd_42762.map.gz | 245.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-42762-v30.xml emd-42762-v30.xml emd-42762.xml emd-42762.xml | 24.2 KB 24.2 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_42762.png emd_42762.png | 209.2 KB | ||
| Filedesc metadata |  emd-42762.cif.gz emd-42762.cif.gz | 9.5 KB | ||
| その他 |  emd_42762_additional_1.map.gz emd_42762_additional_1.map.gz | 254.3 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42762 http://ftp.pdbj.org/pub/emdb/structures/EMD-42762 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42762 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42762 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_42762_validation.pdf.gz emd_42762_validation.pdf.gz | 512.5 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_42762_full_validation.pdf.gz emd_42762_full_validation.pdf.gz | 512.1 KB | 表示 | |
| XML形式データ |  emd_42762_validation.xml.gz emd_42762_validation.xml.gz | 8 KB | 表示 | |
| CIF形式データ |  emd_42762_validation.cif.gz emd_42762_validation.cif.gz | 9.3 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42762 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42762 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42762 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42762 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8uxfMC  8uq2C  8uq3C  8uq4C  8uq5C  8uxcC  8uxeC  8uxgC  8uxhC  8uxiC  8uxlC  8uxmC C: 同じ文献を引用 ( M: このマップから作成された原子モデル |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_42762.map.gz / 形式: CCP4 / 大きさ: 512 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_42762.map.gz / 形式: CCP4 / 大きさ: 512 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Composite map of the Structure of PKA phosphorylated human RyR2-R420W in the primed state | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.8415 Å | ||||||||||||||||||||||||||||||||||||
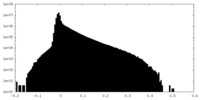
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-追加マップ: Raw consensus map of the Structure of PKA...
| ファイル | emd_42762_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Raw consensus map of the Structure of PKA phosphorylated human RyR2-R420W in the primed state | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
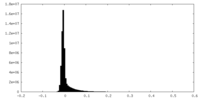
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Complex of RyR2-R420W and Calstabin-2
| 全体 | 名称: Complex of RyR2-R420W and Calstabin-2 |
|---|---|
| 要素 |
|
-超分子 #1: Complex of RyR2-R420W and Calstabin-2
| 超分子 | 名称: Complex of RyR2-R420W and Calstabin-2 / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#2 |
|---|
-超分子 #2: Ryanodine Receptor 2
| 超分子 | 名称: Ryanodine Receptor 2 / タイプ: complex / ID: 2 / 親要素: 1 / 含まれる分子: #1 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-超分子 #3: Peptidyl- cis-trans isomerase FKBP1B
| 超分子 | 名称: Peptidyl- cis-trans isomerase FKBP1B / タイプ: complex / ID: 3 / 親要素: 1 / 含まれる分子: #2 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Ryanodine receptor 2
| 分子 | 名称: Ryanodine receptor 2 / タイプ: protein_or_peptide / ID: 1 / コピー数: 4 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 565.315125 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MADGGEGEDE IQFLRTDDEV VLQCTATIHK EQQKLCLAAE GFGNRLCFLE STSNSKNVPP DLSICTFVLE QSLSVRALQE MLANTVEKS EGQVDVEKWK FMMKTAQGGG HRTLLYGHAI LLRHSYSGMY LCCLSTSRSS TDKLAFDVGL QEDTTGEACW W TIHPASKQ ...文字列: MADGGEGEDE IQFLRTDDEV VLQCTATIHK EQQKLCLAAE GFGNRLCFLE STSNSKNVPP DLSICTFVLE QSLSVRALQE MLANTVEKS EGQVDVEKWK FMMKTAQGGG HRTLLYGHAI LLRHSYSGMY LCCLSTSRSS TDKLAFDVGL QEDTTGEACW W TIHPASKQ RSEGEKVRVG DDLILVSVSS ERYLHLSYGN GSLHVDAAFQ QTLWSVAPIS SGSEAAQGYL IGGDVLRLLH GH MDECLTV PSGEHGEEQR RTVHYEGGAV SVHARSLWRL ETLRVAWSGS HIRWGQPFRL RHVTTGKYLS LMEDKNLLLM DKE KADVKS TAFTFRSSKE KLDVGVRKEV DGMGTSEIKY GDSVCYIQHV DTGLWLTYQS VDVKSVRMGS IQRKAIMHHE GHMD DGISL SRSQHEESRT ARVIWSTVFL FNRFIRGLDA LSKKAKASTV DLPIESVSLS LQDLIGYFHP PDEHLEHEDK QNRLR ALKN RQNLFQEEGM INLVLECIDR LHVYSSAAHF ADVAGREAGE SWKSILNSLY ELLAALIRGN RKNCAQFSGS LDWLIS RLE RLEASSGILE VLHCVLVESP EALNIIKEGH IKSIISLLDK HGRNHKVLDV LCSLCVCHGV AVRSNQHLIC DNLLPGR DL LLQTRLVNHV SSMRPNIFLG VSEGSAQYKK WYYELMVDHT EPFVTAEATH LRVGWASTEG YSPYPGGGEE WGGNGVGD D LFSYGFDGLH LWSGCIARTV SSPNQHLLRT DDVISCCLDL SAPSISFRIN GQPVQGMFEN FNIDGLFFPV VSFSAGIKV RFLLGGRHGE FKFLPPPGYA PCYEAVLPKE KLKVEHSREY KQERTYTRDL LGPTVSLTQA AFTPIPVDTS QIVLPPHLER IREKLAENI HELWVMNKIE LGWQYGPVRD DNKRQHPCLV EFSKLPEQER NYNLQMSLET LKTLLALGCH VGISDEHAED K VKKMKLPK NYQLTSGYKP APMDLSFIKL TPSQEAMVDK LAENAHNVWA RDRIRQGWTY GIQQDVKNRR NPRLVPYTLL DD RTKKSNK DSLREAVRTL LGYGYNLEAP DQDHAARAEV CSGTGERFRI FRAEKTYAVK AGRWYFEFET VTAGDMRVGW SRP GCQPDQ ELGSDERAFA FDGFKAQRWH QGNEHYGRSW QAGDVVGCMV DMNEHTMMFT LNGEILLDDS GSELAFKDFD VGDG FIPVC SLGVAQVGRM NFGKDVSTLK YFTICGLQEG YEPFAVNTNR DITMWLSKRL PQFLQVPSNH EHIEVTRIDG TIDSS PCLK VTQKSFGSQN SNTDIMFYRL SMPIECAEVF SKTVAGGLPG AGLFGPKNDL EDYDADSDFE VLMKTAHGHL VPDRVD KDK EATKPEFNNH KDYAQEKPSR LKQRFLLRRT KPDYSTSHSA RLTEDVLADD RDDYDFLMQT STYYYSVRIF PGQEPAN VW VGWITSDFHQ YDTGFDLDRV RTVTVTLGDE KGKVHESIKR SNCYMVCAGE SMSPGQGRNN NGLEIGCVVD AASGLLTF I ANGKELSTYY QVEPSTKLFP AVFAQATSPN VFQFELGRIK NVMPLSAGLF KSEHKNPVPQ CPPRLHVQFL SHVLWSRMP NQFLKVDVSR ISERQGWLVQ CLDPLQFMSL HIPEENRSVD ILELTEQEEL LKFHYHTLRL YSAVCALGNH RVAHALCSHV DEPQLLYAI ENKYMPGLLR AGYYDLLIDI HLSSYATARL MMNNEYIVPM TEETKSITLF PDENKKHGLP GIGLSTSLRP R MQFSSPSF VSISNECYQY SPEFPLDILK SKTIQMLTEA VKEGSLHARD PVGGTTEFLF VPLIKLFYTL LIMGIFHNED LK HILQLIE PSVFKEAATP EEESDTLEKE LSVDDAKLQG AGEEEAKGGK RPKEGLLQMK LPEPVKLQMC LLLQYLCDCQ VRH RIEAIV AFSDDFVAKL QDNQRFRYNE VMQALNMSAA LTARKTKEFR SPPQEQINML LNFKDDKSEC PCPEEIRDQL LDFH EDLMT HCGIELDEDG SLDGNSDLTI RGRLLSLVEK VTYLKKKQAE KPVESDSKKS STLQQLISET MVRWAQESVI EDPEL VRAM FVLLHRQYDG IGGLVRALPK TYTINGVSVE DTINLLASLG QIRSLLSVRM GKEEEKLMIR GLGDIMNNKV FYQHPN LMR ALGMHETVME VMVNVLGGGE SKEITFPKMV ANCCRFLCYF CRISRQNQKA MFDHLSYLLE NSSVGLASPA MRGSTPL DV AAASVMDNNE LALALREPDL EKVVRYLAGC GLQSCQMLVS KGYPDIGWNP VEGERYLDFL RFAVFCNGES VEENANVV V RLLIRRPECF GPALRGEGGN GLLAAMEEAI KIAEDPSRDG PSPNSGSSKT LDTEEEEDDT IHMGNAIMTF YSALIDLLG RCAPEMHLIH AGKGEAIRIR SILRSLIPLG DLVGVISIAF QMPTIAKDGN VVEPDMSAGF CPDHKAAMVL FLDRVYGIEV QDFLLHLLE VGFLPDLRAA ASLDTAALSA TDMALALNRY LCTAVLPLLT RCAPLFAGTE HHASLIDSLL HTVYRLSKGC S LTKAQRDS IEVCLLSICG QLRPSMMQHL LRRLVFDVPL LNEHAKMPLK LLTNHYERCW KYYCLPGGWG NFGAASEEEL HL SRKLFWG IFDALSQKKY EQELFKLALP CLSAVAGALP PDYMESNYVS MMEKQSSMDS EGNFNPQPVD TSNITIPEKL EYF INKYAE HSHDKWSMDK LANGWIYGEI YSDSSKVQPL MKPYKLLSEK EKEIYRWPIK ESLKTMLAWG WRIERTREGD SMAL YNRTR RISQTSQVSV DAAHGYSPRA IDMSNVTLSR DLHAMAEMMA ENYHNIWAKK KKMELESKGG GNHPLLVPYD TLTAK EKAK DREKAQDILK FLQINGYAVS RGFKDLELDT PSIEKRFAYS FLQQLIRYVD EAHQYILEFD GGSRGKGEHF PYEQEI KFF AKVVLPLIDQ YFKNHRLYFL SAASRPLCSG GHASNKEKEM VTSLFCKLGV LVRHRISLFG NDATSIVNCL HILGQTL DA RTVMKTGLES VKSALRAFLD NAAEDLEKTM ENLKQGQFTH TRNQPKGVTQ IINYTTVALL PMLSSLFEHI GQHQFGED L ILEDVQVSCY RILTSLYALG TSKSIYVERQ RSALGECLAA FAGAFPVAFL ETHLDKHNIY SIYNTKSSRE RAALSLPTN VEDVCPNIPS LEKLMEEIVE LAESGIRYTQ MPHVMEVILP MLCSYMSRWW EHGPENNPER AEMCCTALNS EHMNTLLGNI LKIIYNNLG IDEGAWMKRL AVFSQPIINK VKPQLLKTHF LPLMEKLKKK AATVVSEEDH LKAEARGDMS EAELLILDEF T TLARDLYA FYPLLIRFVD YNRAKWLKEP NPEAEELFRM VAEVFIYWSK SHNFKREEQN FVVQNEINNM SFLITDTKSK MS KAAVSDQ ERKKMKRKGD RYSMQTSLIV AALKRLLPIG LNICAPGDQE LIALAKNRFS LKDTEDEVRD IIRSNIHLQG KLE DPAIRW QMALYKDLPN RTDDTSDPEK TVERVLDIAN VLFHLEQKSK RVGRRHYCLV EHPQRSKKAV WHKLLSKQRK RAVV ACFRM APLYNLPRHR AVNLFLQGYE KSWIETEEHY FEDKLIEDLA KPGAEPPEED EGTKRVDPLH QLILLFSRTA LTEKC KLEE DFLYMAYADI MAKSCHDEED DDGEEEVKSF EEKEMEKQKL LYQQARLHDR GAAEMVLQTI SASKGETGPM VAATLK LGI AILNGGNSTV QQKMLDYLKE KKDVGFFQSL AGLMQSCSVL DLNAFERQNK AEGLGMVTEE GSGEKVLQDD EFTCDLF RF LQLLCEGHNS DFQNYLRTQT GNNTTVNIII STVDYLLRVQ ESISDFYWYY SGKDVIDEQG QRNFSKAIQV AKQVFNTL T EYIQGPCTGN QQSLAHSRLW DAVVGFLHVF AHMQMKLSQD SSQIELLKEL MDLQKDMVVM LLSMLEGNVV NGTIGKQMV DMLVESSNNV EMILKFFDMF LKLKDLTSSD TFKEYDPDGK GVISKRDFHK AMESHKHYTQ SETEFLLSCA ETDENETLDY EEFVKRFHE PAKDIGFNVA VLLTNLSEHM PNDTRLQTFL ELAESVLNYF QPFLGRIEIM GSAKRIERVY FEISESSRTQ W EKPQVKES KRQFIFDVVN EGGEKEKMEL FVNFCEDTIF EMQLAAQISE SDLNERSANK EESEKERPEE QGPRMAFFSI LT VRSALFA LRYNILTLMR MLSLKSLKKQ MKKVKKMTVK DMVTAFFSSY WSIFMTLLHF VASVFRGFFR IICSLLLGGS LVE GAKKIK VAELLANMPD PTQDEVRGDG EEGERKPLEA ALPSEDLTDL KELTEESDLL SDIFGLDLKR EGGQYKLIPH NPNA GLSDL MSNPVPMPEV QEKFQEQKAK EEEKEEKEET KSEPEKAEGE DGEKEEKAKE DKGKQKLRQL HTHRYGEPEV PESAF WKKI IAYQQKLLNY FARNFYNMRM LALFVAFAIN FILLFYKVST SSVVEGKELP TRSSSENAKV TSLDSSSHRI IAVHYV LEE SSGYMEPTLR ILAILHTVIS FFCIIGYYCL KVPLVIFKRE KEVARKLEFD GLYITEQPSE DDIKGQWDRL VINTQSF PN NYWDKFVKRK VMDKYGEFYG RDRISELLGM DKAALDFSDA REKKKPKKDS SLSAVLNSID VKYQMWKLGV VFTDNSFL Y LAWYMTMSVL GHYNNFFFAA HLLDIAMGFK TLRTILSSVT HNGKQLVLTV GLLAVVVYLY TVVAFNFFRK FYNKSEDGD TPDMKCDDML TCYMFHMYVG VRAGGGIGDE IEDPAGDEYE IYRIIFDITF FFFVIVILLA IIQGLIIDAF GELRDQQEQV KEDMETKCF ICGIGNDYFD TVPHGFETHT LQEHNLANYL FFLMYLINKD ETEHTGQESY VWKMYQERCW EFFPAGDCFR K QYEDQLN UniProtKB: Ryanodine receptor 2 |
-分子 #2: Peptidyl-prolyl cis-trans isomerase FKBP1B
| 分子 | 名称: Peptidyl-prolyl cis-trans isomerase FKBP1B / タイプ: protein_or_peptide / ID: 2 / コピー数: 4 / 光学異性体: LEVO / EC番号: peptidylprolyl isomerase |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 11.798501 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MGVEIETISP GDGRTFPKKG QTCVVHYTGM LQNGKKFDSS RDRNKPFKFR IGKQEVIKGF EEGAAQMSLG QRAKLTCTPD VAYGATGHP GVIPPNATLI FDVELLNLE UniProtKB: Peptidyl-prolyl cis-trans isomerase FKBP1B |
-分子 #3: ZINC ION
| 分子 | 名称: ZINC ION / タイプ: ligand / ID: 3 / コピー数: 4 / 式: ZN |
|---|---|
| 分子量 | 理論値: 65.409 Da |
-分子 #4: ADENOSINE-5'-TRIPHOSPHATE
| 分子 | 名称: ADENOSINE-5'-TRIPHOSPHATE / タイプ: ligand / ID: 4 / コピー数: 8 / 式: ATP |
|---|---|
| 分子量 | 理論値: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 2.5 mg/mL | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 7.4 構成要素:
| ||||||||||||||||||||||||
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 温度 | 最低: 80.0 K / 最高: 100.0 K |
| 特殊光学系 | エネルギーフィルター - 名称: GIF Bioquantum / エネルギーフィルター - スリット幅: 20 eV |
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) デジタル化 - サイズ - 横: 5760 pixel / デジタル化 - サイズ - 縦: 4092 pixel / 平均電子線量: 58.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 100.0 µm / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 1.2 µm / 最小 デフォーカス(公称値): 0.5 µm |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: INSILICO MODEL / In silico モデル: CryoSPARC ab initio |
|---|---|
| 最終 再構成 | 想定した対称性 - 点群: C4 (4回回転対称) / 解像度のタイプ: BY AUTHOR / 解像度: 3.13 Å / 解像度の算出法: FSC 0.143 CUT-OFF / ソフトウェア - 名称: cryoSPARC / 使用した粒子像数: 102478 |
| 初期 角度割当 | タイプ: MAXIMUM LIKELIHOOD / ソフトウェア - 名称: cryoSPARC |
| 最終 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
 ムービー
ムービー コントローラー
コントローラー
















































































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)